Abstract
AIM: To assess the efficacy of combined transcatheter arterial chemoembolization (TACE) and percutaneous microwave coagulation therapy (PMCT) for small hepatocellular carcinoma (HCC).
METHODS: Thirty-five patients with a total of 41 HCC nodules (≤ 3 cm in diameter) were treated with TACE followed by computed tomograghy (CT)-guided percutaneous microwave coagulation therapy (PMCT) within 1-3 wk.
RESULTS: By biopsies and enhanced CT scans, complete necrosis of the tumor and 3-5 mm of the surrounding non-cancerous area were observed in 34 foci. In seven foci, incomplete necrosis of the surrounding parenchyma was observed. Serum alpha-fetoprotein (AFP) levels returned to normal 10 d after treatment in 25 patients who originally had high serum AFP levels. The follow-up period was 6-31 mo, and all patients remained alive. One patient had a recurrence in the subsegments of the liver, and another patient had a recurrence near the original lesion.
CONCLUSION: Combined therapy with TACE and PMCT is a safe and effective treatment without severe complications for small HCC.
Keywords: Liver neoplasms, Therapy, Hepatocellular carcinoma, Transcatheter arterial chemoembolization, Microwave coagulation therapy, Percutaneous local treatment
INTRODUCTION
Surgical resection[1], transcatheter arterial chemoem-bolization (TACE), and percutaneous ethanol injection (PEI)[2–4] are effective therapies for small hepatocellular carcinoma (HCC). However, for patients with poor liver functions, hepatectomy is not the first treatment choice. TACE is not effective for HCC with a poor blood supply[5], and PEI only yields incomplete necrosis of the tumor due to the uneven distribution of ethanol[6]. Although percutaneous microwave coagulation therapy (PMCT) can lead to complete necrosis of the hepatocarcinoma cells, the induced area of necrosis is small[7,8]. TACE has the advantage of reducing the local blood supply of the tumor foci, resulting in tissue necrosis and inflammatory edema. Therefore, it can decrease the cooling effect of blood flow on the heating action of microwaves, and enhance the coagulation action of microwaves[9]. In the present study, we investigate the therapeutic efficacy of the combied therapy of TACE and PMCT for the treatment of small HCC.
MATERIALS AND METHODS
Patient data
A total of 35 patients received the combined therapy of TACE and PMCT in our hospital between April 2005 and May 2007. These patients comprised 27 males and eight females, with an age range of 27-78 years, and a mean age of 56.69 ± 14.02 years. All patients had a history of hepatitis B and liver cirrhosis, with liver functions categorized as class A by Child-Pugh classification. All of the cases met the diagnostic criteria of primary HCC. Twenty-eight cases were confirmed by pathological examination of puncture-biopsied specimens under the guidance of computed tomograghy (CT) before PMCT. Twenty-five cases had elevated levels of serum alpha-fetoprotein (AFP). The size of the tumor focus was determined by measurement of the largest and shortest diameters of the largest section. All tumor foci were less than 3 cm in diameter.
Therapeutic methods
All patients were initially treated with TACE. Hepatic artery angiography was performed using the Seldinger technique. Femoral arterial catheterization was conducted through the common hepatic artery or proper hepatic artery, and the location, number, size, and blood supply of the tumors were evaluated. Subsequently, a microcatheter was super-selectively inserted into the hepatic lobe or hepatic segmental artery branch, and mixed suspensions of iodized oil (3-6 mL) and epirubicin (20-30 mg) were infused into the artery through the catheter. Finally, gelatin sponge particles were infused to embolize the artery until the arterial blood flow supplying the tumor was completely blocked.
PMCT was initiated 1-3 wk after TACE. The microwave therapeutic device, FORSEA MTC-3 for inter-tissue tumor treatment, was produced by Nanjing Qinghai Microwave Electric Institute (Nanjing, China). The diameter of its electrode needle was 14 G, and the instrument was cooled by a cold water cycling system. To relieve pain in the patients, dolantin (100 mg) and valium (10 mg) were injected intramuscularly 5 min before the PMCT.
The puncture site and pathway were determined under the guidance of CT. After anesthetizing the puncture site with 2% lidocaine, a 12 G guide-needle was placed at its opposite side, across the tumor focus. The electrode needle of the microwave device was connected to the output machine of the microwave instrument via a flexible coaxial cable, and the cooling water tube was connected. The constant-flow pump was switched on to test the functioning of the cold water cycling system. The inner needle of the guide-needle was withdrawn, and the electrode needle of the microwave machine was inserted through the outer needle of the guide to place the electrode in the tumor area. The microwave power was set at 60-70 W. The coagulation time for each focus was 10-15 min, and the coagulation area covering the tumor focus and its surrounding area measured 5 mm or more. If a single needle coagulation treatment did not produce satisfactory results, a second PMCT therapy was conducted a week later.
After TACE and PMCT treatment, liver protection, anti-inflammatory and sedation therapies were prescribed. A follow-up study by repeat CT (plain and enhanced) and serum AFP level measurement was conducted once every 1-2 mo.
RESULTS
One to three weeks after all 41 foci were treated with TACE, these foci received PMCT treatment once. Among them, six foci were treated with a second PMCT within 1 wk.
Pathological examinations of biopsied specimens and enhanced CT after PMCT showed complete tumor necrosis in 34 foci, together with complete ring-shaped necrosis of the surrounding non-cancerous hepatic parenchymal tissue, measuring 3-5 mm in width (Figure 1). Seven other foci showed complete necrosis of the tumor, with incomplete necrosis of the surrounding non-cancerous hepatic parenchyma.
Figure 1.
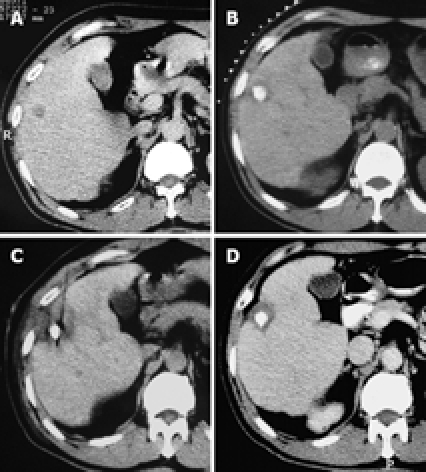
Pathological examinations of biopsied specimens and enhanced CT after PMCT showed complete tumor necrosis in 34 foci, together with complete ring-shaped necrosis of the surrounding non-cancerous hepatic parenchymal tissue, measuring 3-5 mm in width. A: CT scan of a primary HCC in the right anterior lobe of the liver, with a diameter of 1.6 cm × 1.4 cm; B: After TACE, the accumulation of iodized oil in the tumor area was satisfactory; C: PMCT was initiated 17 d later; D: One month after PMCT, an enhanced CT scan showed a complete non-enhanced area of the tumor, and a non-cancerous ring-shaped area surrounding the tumor (measuring 4-5 mm), indicating complete necrosis of the tumor lesion.
Among the 35 patients, serum AFP levels were elevated in 25; the AFP levels in these 25 patients returned to normal within 10 d after treatment.
Post-embolization symptoms included fever, pain, nausea, and vomiting after TACE, all of which were relieved by anti-inflammatory, liver protection, and sedation treatment. In the process of PMCT, most patients complained of local burning pain, which was generally tolerable, but in some patients, the microwave coagulation therapy had to be carried out intermittently. After PMCT, some patients reported having fever and local pain, but these were not severe. Complications such as hemorrhage, subcapsular hematoma of the liver, or biliary duct damage were not found, nor was local dissemination of cancer cells along the puncture needle pathway.
All patients were followed up for 6-31 mo. No recurrence was found in 33 patients. One patient had a gradual increase of serum AFP levels 10 mo after the treatment, but repeat CT and magnetic resonance imaging (MRI) examinations revealed no recurrence. However, hepatic arteriography detected new tumor foci in the segments of the liver. Another patient showed an elevation of serum AFP levels 8 mo after treatment, and a CT scan showed recurrence in the lateral side of the original focus. The recurring foci were all treated with TACE and PMCT again. All patients remained alive during the follow-up period.
DISCUSSION
The mechanism of PMCT for HCC is based on the heating effect of microwaves and the sensitivity of the tumor to the heating action. The high temperature produced by the electrodes inserted into the tumor tissue results in the rapid coagulation and necrosis of the tumor tissue, thus achieving the goal of destroying the tumor. The area of PMCT is related to the diameter and number of the microwave electrode, the output power of the machine, coagulation time, and local blood circulation[7,10–13]. Increases in the diameter of the electrodes, the output power, and the coagulation time may enlarge the treatment areas of PMCT. However, increasing the diameter of the electrodes may also lead to increased rates of post-puncture hemorrhage, and an extension of the coagulation time may result in more pain for the patients. In practice, the diameter of the electrode and the output power of the microwave therapeutic machine are constant. Therefore, reducing the local blood supply is the only approach to achieving the goal of increasing the PMCT area.
It is well known that primary HCC possesses an abundant blood supply. TACE through the hepatic artery, especially the injection of iodine oil and gelatin sponge particles, blocks the artery supply to the tumor. The iodine oil can fill up the portal vein surrounding the tumor through the communication branches connecting the arterial and portal veins[14,15]. Thus, it can decrease the blood flow volume of the portal vein, and alleviate the adverse cooling effect induced by abundant blood flow[16,17]. In addition, TACE can result in ischemia of the tumor tissue and inflammatory edema, which accelerates tumor necrosis and enhances the coagulation effect of microwaves[18]. This forms the theoretical basis for the combined therapy of transcatheter hepatic artery chemoembolization with percutaneous microwave ablation for small HCC. Ishida et al[19] used hepatic artery embolization combined with a temporal block of the hepatic vein to reduce the segmental blood supply to the tumor. The therapeutic range of PMCT was increased; however, the procedures were too complicated to be practiced often.
Primary HCC is a malignancy that usually develops from liver cirrhosis, and is likely to have multiple foci. Although modern advanced diagnostic imaging techniques (spiral CT and MRI) can detect tumor nodules smaller than 1 cm, the detection rate is quite low. TACE therapy prior to PMCT enhances the efficacy of the latter. In addition, TACE can also help detect nodules that are undetectable by conventional imaging techniques, because digital subtraction angiography and the so-called iodine oil-CT are still the best methods for the detection of small HCC. Another advantage of TACE therapy prior to PMCT is that TACE can quickly and effectively control the foci that are inaccessible to PMCT.
Seki et al[20] treated 18 cases of small HCC of less than 3 cm using PMCT, and achieved complete necrosis in 17 of them. After a follow-up of 12-31 mo, all the patients were still alive. Lu et al[21] treated 67 cases of small HCC of less than 3 cm using the microwave therapy device produced by Nanjing Qinghai Company (Nanjing, China), and achieved a complete necrosis rate of 94% (63 cases). We used a combined therapy of transcatheter hepatic artery chemoembolization with PMCT for 35 cases of small HCC, and achieved complete necrosis in all cases. After a follow-up of 6-31 mo, only one patient had a recurrence at the original tumor site. Liang et al[22] analyzed the correlative factors for the prognosis of 288 HCC patients who received PMCT. They concluded that HCC patients with a single focus of a diameter ≤ 4 cm, and a liver function of Child-Pugh class A can have long-term survival after PMCT. Therefore, PMCT is ideal for small HCC. It can completely substitute for surgical resection, especially for elderly patients or those with a poor general condition or poor liver function[23].
Pathological examinations indicate that HCC is very likely to invade the capsule or extracapsular tissues[24,25]. Therefore, to achieve the goal of complete necrosis of tumor tissue and to increase its overall therapeutic efficacy, PMCT must not only destroy the tumor cells in the center of the tumor, but also destroy the adjacent non-cancerous hepatic parenchyma tissue. The microwave therapy device used in our study could produce coagulative tissue necrosis with a diameter of 4-5 cm under the conditions of an output power of 60-70 W, and a coagulation time of 15 min. By TACE, the area of tissue necrosis using a single needle electrode was even larger, completely covering a liver cancer nodule of ≤ 3 cm, with a tumor-free margin of 5 mm. Among our patients, there was one case of recurrence at the original tumor site, with incomplete liver necrosis of the surrounding non-cancerous liver parenchyma. This was probably due to the invasion of the tumor into the capsule or subcapsular tissues, or an insufficient coagulation time, which resulted in the incomplete killing of the tumor cells.
Compared with ultrasound-guided treatment, the present study was carried out under guidance of CT and has multiple advantages[26,27]. It allows for determination of the precise locations of the foci, especially after TACE because iodine oil accumulation in the tumor foci makes the identification of their location and range easier. Additionally, when a plain CT scan is unable to clearly show the location of a tumor due to its small size, CT-guided puncturing of the focus can be performed based on the result of the MRI scan, thus reducing the “blindness” of the therapy. Finally, CT-guided liver puncturing has almost no “blind area”, and is not affected by the adjacent organ full of gas.
Only mild adverse reactions were reported by our patients treated by PMCT. Local pain, during and after the treatment, was not very severe. No internal bleeding, subcapsular hematoma, biliary duct damage, or spreading of the tumor along the puncturing pathway were found. This suggests that PMCT is a safe therapy. Our results show that the combined TACE and PMCT for small HCC (≤ 3 cm in diameter) is minimally invasive, simple and efficacious. Although further studies are needed, this combined therapy appears to be the first choice of treatment for small HCC that is unsuitable for surgical resection.
COMMENTS
Background
Surgical resection, transcatheter arterial chemoembolization (TACE), and percutaneous ethanol injection (PEI) are effective therapies for small hepatocellular carcinoma (HCC). However, for patients with poor liver functions, hepatectomy is not the first treatment choice. TACE is not effective for HCC with a poor blood supply, and PEI only yields incomplete necrosis of the tumor due to the uneven distribution of ethanol.
Research frontiers
TACE has the advantage of reducing the local blood supply of the tumor foci, resulting in tissue necrosis and inflammatory edema. Therefore, it can decrease the cooling effect of blood flow on the heating action of microwaves, and enhance the coagulating action of microwaves.
Innovations and breakthroughs
In the present study, the authors investigated the efficacy of the combined TACE and PMCT for small HCC.
Applications
The study shows that combined TACE and PMCT for treatment of small HCC (≤ 3 cm in diameter) is minimally invasive, simple and efficacious. Although further studies are needed, this combined therapy appears to be the first choice of treatment for small HCC that is unsuitable for surgical resection.
Peer review
This is an interesting study which shows the advantages of combined TACE and PMCT for small HCC. It may provide useful information for us.
Footnotes
Peer reviewer: Serdar Karakose, PhD, Professor, Department of Radiology, Meram Medical Faculty, Selcuk University, Konya 42080, Turkey
S- Editor Li JL L- Editor Ma JY E- Editor Yin DH
References
- 1.Wakai T, Shirai Y, Suda T, Yokoyama N, Sakata J, Cruz PV, Kawai H, Matsuda Y, Watanabe M, Aoyagi Y, et al. Long-term outcomes of hepatectomy vs percutaneous ablation for treatment of hepatocellular carcinoma ≤ 4 cm. World J Gastroenterol. 2006;12:546–552. doi: 10.3748/wjg.v12.i4.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shiina S, Tagawa K, Unuma T, Fujino H, Uta Y, Niwa Y, Hata Y, Komatsu Y, Shiratori Y, Terano A. Percutaneous ethanol injection therapy of hepatocellular carcinoma: analysis of 77 patients. AJR Am J Roentgenol. 1990;155:1221–1226. doi: 10.2214/ajr.155.6.2173384. [DOI] [PubMed] [Google Scholar]
- 3.Livraghi T, Bolondi L, Lazzaroni S, Marin G, Morabito A, Rapaccini GL, Salmi A, Torzilli G. Percutaneous ethanol injection in the treatment of hepatocellular carcinoma in cirrhosis. A study on 207 patients. Cancer. 1992;69:925–929. doi: 10.1002/1097-0142(19920215)69:4<925::aid-cncr2820690415>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 4.Shiina S, Tagawa K, Unuma T, Takanashi R, Yoshiura K, Komatsu Y, Hata Y, Niwa Y, Shiratori Y, Terano A. Percutaneous ethanol injection therapy for hepatocellular carcinoma. A histopathologic study. Cancer. 1991;68:1524–1530. doi: 10.1002/1097-0142(19911001)68:7<1524::aid-cncr2820680711>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 5.Kuroda C, Sakurai M, Monden M, Marukawa T, Hosoki T, Tokunaga K, Wakasa K, Okamura J, Kozuka T. Limitation of transcatheter arterial chemoembolization using iodized oil for small hepatocellular carcinoma. A study in resected cases. Cancer. 1991;67:81–86. doi: 10.1002/1097-0142(19910101)67:1<81::aid-cncr2820670116>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 6.Ishii H, Okada S, Nose H, Okusaka T, Yoshimori M, Takayama T, Kosuge T, Yamasaki S, Sakamoto M, Hirohashi S. Local recurrence of hepatocellular carcinoma after percutaneous ethanol injection. Cancer. 1996;77:1792–1796. doi: 10.1002/(SICI)1097-0142(19960501)77:9<1792::AID-CNCR6>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 7.Seki T, Wakabayashi M, Nakagawa T, Imamura M, Tamai T, Nishimura A, Yamashiki N, Okamura A, Inoue K. Percutaneous microwave coagulation therapy for patients with small hepatocellular carcinoma: comparison with percutaneous ethanol injection therapy. Cancer. 1999;85:1694–1702. [PubMed] [Google Scholar]
- 8.Peng QR, Zhang LS, Xie SM, Chen GQ, Duan YX. A study on therapeutical effect of CT-guided percutaneous microwave coagulation therapy (PMCT) for primary liver cancer. Xiandai Xiaohua Ji Jieru Zhenliao. 2005;10:200–202. [Google Scholar]
- 9.Xiao ZY, Chen XP, Huang ZY. Treatment of liver cancer with microwave coagulation after interruption of cancerous blood supply: A clinical analysis of 120 cases. Zhonghua Gandan Waike Zazhi. 2005;11:806–808. [Google Scholar]
- 10.Dong BW, Liang P, Yu XL, Zeng XQ, Wang PJ, Su L, Wang XD, Xin H, Li S. Sonographically guided microwave coagulation treatment of liver cancer: an experimental and clinical study. AJR Am J Roentgenol. 1998;171:449–454. doi: 10.2214/ajr.171.2.9694473. [DOI] [PubMed] [Google Scholar]
- 11.Yu NC, Raman SS, Kim YJ, Lassman C, Chang X, Lu DS. Microwave liver ablation: influence of hepatic vein size on heat-sink effect in a porcine model. J Vasc Interv Radiol. 2008;19:1087–1092. doi: 10.1016/j.jvir.2008.03.023. [DOI] [PubMed] [Google Scholar]
- 12.Hines-Peralta AU, Pirani N, Clegg P, Cronin N, Ryan TP, Liu Z, Goldberg SN. Microwave ablation: results with a 2.45-GHz applicator in ex vivo bovine and in vivo porcine liver. Radiology. 2006;239:94–102. doi: 10.1148/radiol.2383050262. [DOI] [PubMed] [Google Scholar]
- 13.Simon CJ, Dupuy DE, Iannitti DA, Lu DS, Yu NC, Aswad BI, Busuttil RW, Lassman C. Intraoperative triple antenna hepatic microwave ablation. AJR Am J Roentgenol. 2006;187:W333–W340. doi: 10.2214/AJR.05.0804. [DOI] [PubMed] [Google Scholar]
- 14.Kan Z, Sato M, Ivancev K, Uchida B, Hedgpeth P, Lunderquist A, Rosch J, Yamada R. Distribution and effect of iodized poppyseed oil in the liver after hepatic artery embolization: experimental study in several animal species. Radiology. 1993;186:861–866. doi: 10.1148/radiology.186.3.8381552. [DOI] [PubMed] [Google Scholar]
- 15.Chung JW. Transcatheter arterial chemoembolization of hepatocellular carcinoma. Hepatogastroenterology. 1998;45 Suppl 3:1236–1241. [PubMed] [Google Scholar]
- 16.Patterson EJ, Scudamore CH, Owen DA, Nagy AG, Buczkowski AK. Radiofrequency ablation of porcine liver in vivo: effects of blood flow and treatment time on lesion size. Ann Surg. 1998;227:559–565. doi: 10.1097/00000658-199804000-00018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goldberg SN, Hahn PF, Tanabe KK, Mueller PR, Schima W, Athanasoulis CA, Compton CC, Solbiati L, Gazelle GS. Percutaneous radiofrequency tissue ablation: does perfusion-mediated tissue cooling limit coagulation necrosis? J Vasc Interv Radiol. 1998;9:101–111. doi: 10.1016/s1051-0443(98)70491-9. [DOI] [PubMed] [Google Scholar]
- 18.He W, Liang XN, Zhang XR, Jiang XH, Zheng YC, Zhang Y. Evaluation of ultrasound-guided microwave coagulation in combination with transarterial chemoembolization for large hepatic cancers. Zhongguo Weichuang Waike Zazhi. 2005;5:31–33. [Google Scholar]
- 19.Ishida T, Murakami T, Shibata T, Inoue Y, Takamura M, Niinobu T, Sato T, Nakamura H. Percutaneous microwave tumor coagulation for hepatocellular carcinomas with interruption of segmental hepatic blood flow. J Vasc Interv Radiol. 2002;13:185–191. doi: 10.1016/s1051-0443(07)61937-x. [DOI] [PubMed] [Google Scholar]
- 20.Seki T, Tamai T, Nakagawa T, Imamura M, Nishimura A, Yamashiki N, Ikeda K, Inoue K. Combination therapy with transcatheter arterial chemoembolization and percutaneous microwave coagulation therapy for hepatocellular carcinoma. Cancer. 2000;89:1245–1251. [PubMed] [Google Scholar]
- 21.Lu MD, Xu HX, Kuang M, Xie XY, Liu GJ, Xu ZF, Zheng YL, Liang JY. Research of the improved microwave ablation therapy for treatment of hepatocellular carcioma. Zhongguo Shiyong Waike Zazhi. 2004;24:678–680. [Google Scholar]
- 22.Liang P, Dong B, Yu X, Yu D, Wang Y, Feng L, Xiao Q. Prognostic factors for survival in patients with hepatocellular carcinoma after percutaneous microwave ablation. Radiology. 2005;235:299–307. doi: 10.1148/radiol.2351031944. [DOI] [PubMed] [Google Scholar]
- 23.Aramaki M, Kawano K, Ohno T, Sasaki A, Tahara K, Kai S, Iwashita Y, Kitano S. Microwave coagulation therapy for unresectable hepatocellular carcinoma. Hepatogastroenterology. 2004;51:1784–1787. [PubMed] [Google Scholar]
- 24.Nakajima Y, Nagabuchi E, Sato N, Kamiyama T, Matsuoka S, Une Y, Uchino J. [A clinical study of liver resections in patients with small hepatocellular carcinoma less than three centimeter in diameter] Nippon Geka Gakkai Zasshi. 1992;93:1087–1090. [PubMed] [Google Scholar]
- 25.Kanai T, Hirohashi S, Upton MP, Noguchi M, Kishi K, Makuuchi M, Yamasaki S, Hasegawa H, Takayasu K, Moriyama N. Pathology of small hepatocellular carcinoma. A proposal for a new gross classification. Cancer. 1987;60:810–819. doi: 10.1002/1097-0142(19870815)60:4<810::aid-cncr2820600417>3.0.co;2-1. [DOI] [PubMed] [Google Scholar]
- 26.Guan YS, Sun L, Zhou XP, Li X, Zheng XH. Hepatocellular carcinoma treated with interventional procedures: CT and MRI follow-up. World J Gastroenterol. 2004;10:3543–3548. doi: 10.3748/wjg.v10.i24.3543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang N, Yang WZ, Jiang N, Zheng QB, Huang JY. Treatment of portal vein tumor emboli of hepatocellular carcinoma with CT-guided percutaneous ethanol injection. Jieru Fangshexue Zazhi. 2006;15:670–672. [Google Scholar]


