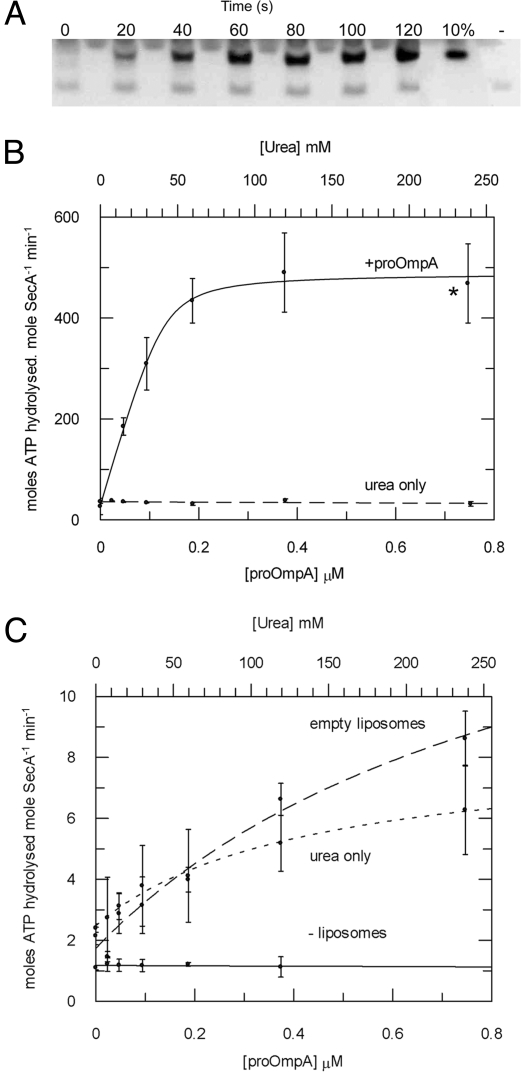
Fig. 2.
Steady-state analysis of the translocation-coupled ATPase activity stimulated by membrane-bound SecYEG and proOmpA. (A) Aliquots of an ATPase assay described below were stopped at 20-s intervals and the amount of successfully translocated proOmpA was analyzed by protease protection and Western blot. These were compared with a measure of 10% of the total proOmpA in each reaction and a negative control carried out in the absence of ATP (−). (B) The ATPase activity of SecA (0.05 μM) was measured in the presence of 1 mM ATP in TKM buffer with 1 μM SecYEG proteoliposomes in TKM buffer, and increasing concentrations of proOmpA in urea (solid line); a control experiment applied the same concentrations of urea without proOmpA (dashed line). The datasets were fitted according to Eq. 3 (SI Equations) to reveal values for Kd,app (Table 1). *, the conditions used to define the Michaelis–Menten parameters for ATP hydrolysis (Table 2). (C) The ATPase activity of SecA in the presence of proOmpA was measured as in B in the absence of any lipids (solid line), in the presence of empty liposomes (dashed line), and with urea only, in the presence of empty liposomes (dotted line).