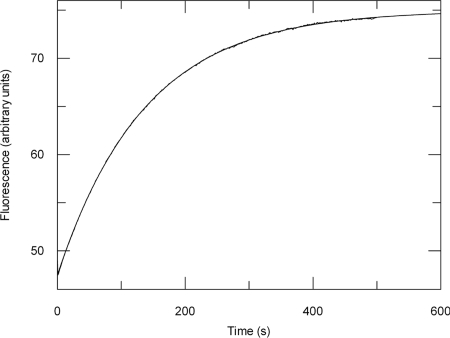
Fig. 4.
The rate constant for ADP release by SecA (koff[ADP]) is equal to the steady-state ATPase rate (kcat). koff[ADP] was measured by displacement of ADP bound to SecA by MANT-ADP, resulting in an increase in FRET between the intrinsic tryptophans in SecA and the MANT fluorophore. SecA (0.5 μM) preincubated with 2.5 μM ADP was mixed with 12.5 μM MANT-ADP in a stopped-flow device and the fluorescence change was measured using excitation light at 296 nm and a 399-nm emission cut-off filter. The data were fitted to a single exponential equation (Eq. 4), giving a koff[ADP] of 0.0073 ± 1.1 × 10−5 s−1.