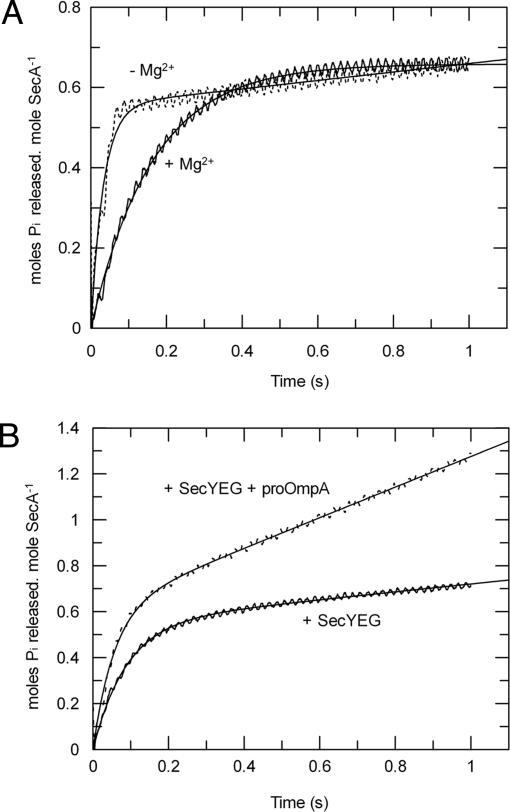
Fig. 5.
Control of the ATPase activity has only a minor contribution from the rate of ATP cleavage. The pre-steady-state ATPase activity of SecA was measured by stopped-flow rapid mixing using Phosphate Sensor. ATP hydrolysis by SecA was measured in isolation with 1 mM ATP in the presence (solid line) or absence (dotted line) of 2 mM MgCl2 (A) and in the presence of 1 mM ATP, 2 mM MgCl2, and 1 μM SecYEG reconstituted into proteoliposomes, with (solid line) or without (dotted line) the addition of 0.75 μM translocation substrate proOmpA (B). The data were fitted to Eq. 4 (SI Equations); the resulting values of ATP cleavage rate (kcleave) are shown in Table 2.