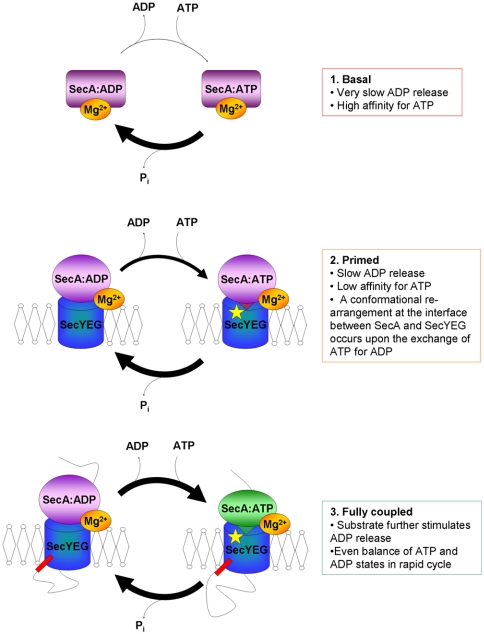
Fig. 6.
Schematic overview depicting the changes in steady-state complex of SecA during the translocation reaction. Both SecA and SecYEG are shown as single objects (which may correspond to dimers). Soluble SecA with Mg2+ bound to an allosteric binding site has low catalytic activity and the dominant steady-state complex is SecA:ADP. When SecA associates with the membrane, SecYEG can alleviate this inhibition, yet ADP release is still relatively slow. The presence of a preprotein substrate renders the system fully active: The apparent affinity for ATP remains the same but ADP release is stimulated, resulting in approximately equal amounts of SecA:ADP and SecA:ATP that are rapidly interconverting through the steady-state cycle. The binding of ATP by SecA (green) causes a change in the conformation at the SecA-YEG interface resulting in insertion of a loop of SecA into the channel in SecY (K268, yellow star) (21, 25). Hydrolysis of ATP brings about retraction of the loop of SecA from the SecYEG channel entrance.