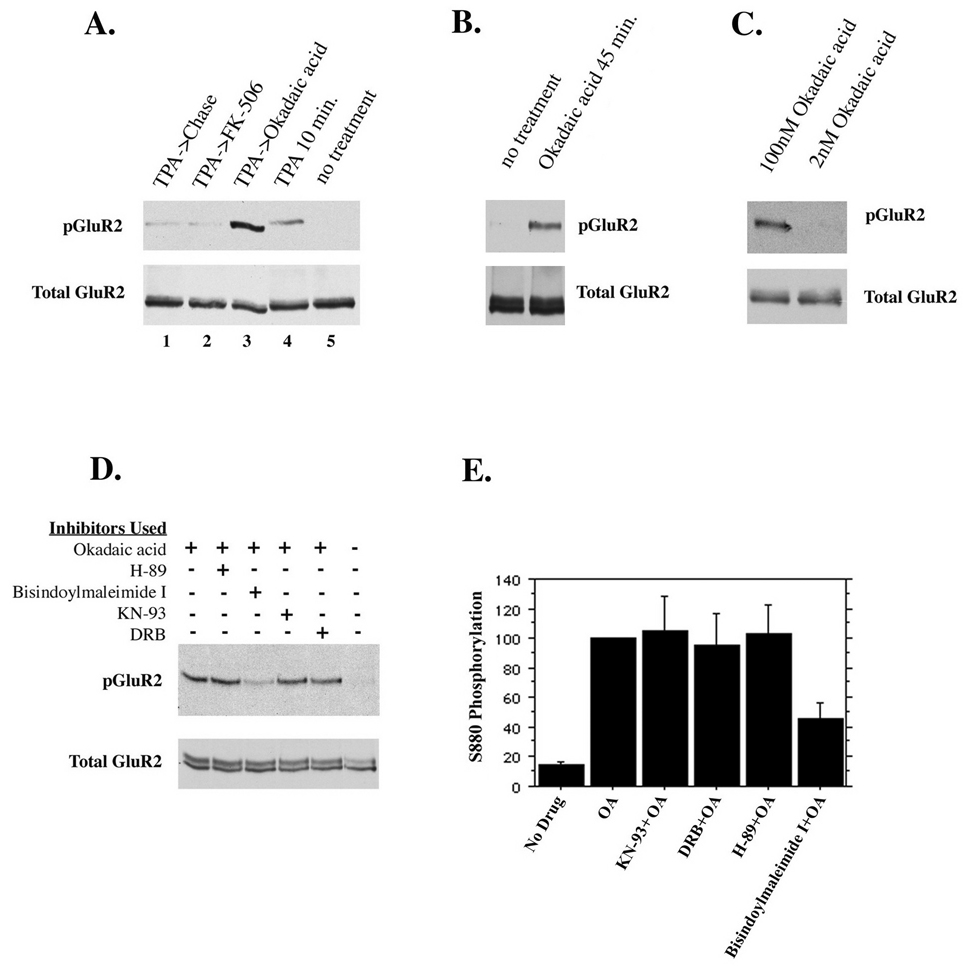
Figure 6. Endogenous regulation of GluR2 phosphorylation.
(A) Cultured hippocampal neurons were treated for 10 minutes with TPA in the presence of 1µM FK-506 (to inhibit PP2B) or 100nM okadaic acid (to inhibit PP1 and PP2A) or without phosphatase inhibitors. Neurons were then chased for 1 hour in the absence or presence of the phosphatase inhibitors as indicated. (B) Inhibition of PP1 and PP2A with okadaic acid (100nM) for 45 minutes reveals an endogenous kinase activity which phosphorylates GluR2. (C) PP2A alone or PP2A and PP1 were inhibited with 2nM and 100nM okadaic acid for 45 minutes, respectively. Inhibition of PP2A alone did not increase GluR2 phosphorylation, indicating that PP1 dephosphorylates GluR2. (D and E) To identify the endogenous kinase activity, different cell-permeable serine/threonine kinase inhibitors were used in the presence of okadaic acid for 45 minutes. Control groups either were untreated or treated with okadaic acid but no kinase inhibitors. Kinase inhibitors used were 2µM H-89 (inhibits PKA), 1µM bisindolylmaleimide I (inhibits PKC), 7.4µM KN-93 (inhibits CaMKII), 100µM 5,6 dichloro-1-beta-D-ribofuranosylbenzimidazole I or DRB (inhibits casein kinase II). Only inhibition of PKC diminished phosphorylation of GluR2 in the presence of okadaic acid (45% of controls treated with okadaic acid but no kinase inhibitor).