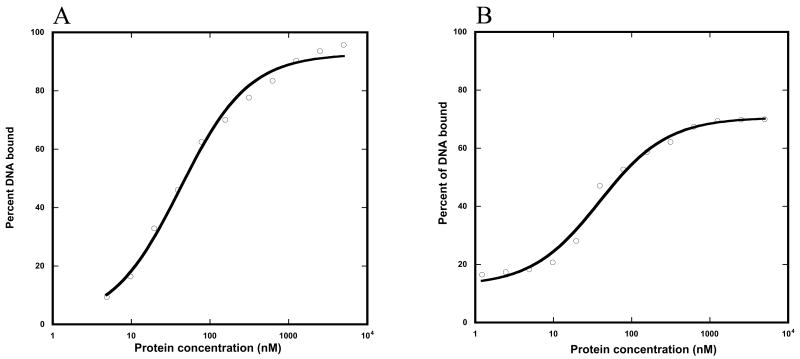
Figure 5. DNA dissociation constants (KD) of WT and the ENEYP variant.
A) WT and B) ENEYP. As described in Materials and Methods, thirteen protein concentrations ranging from 5 uM to 1.2 nM were prepared to cover the range of possible binding constants. Each protein concentration was incubated with 0.1 nM of 3′-recessed DNA substrate in gel-shift buffer, the content of which is listed in Materials and Methods. After the protein-DNA cocktail was mixed, each reaction was incubated at room temperature for 15 minutes. Reaction samples were resolved on a 6% acrylamide non-denaturing gel. The gel was dried bound DNA was quantified using a Phosphoimager as described in Materials and Methods. The KD was calculated by fitting protein concentrations and the corresponding bound DNA fraction to gel-shift equation. X-axis is protein concentration in nM and y-axis is percent of DNA bound.