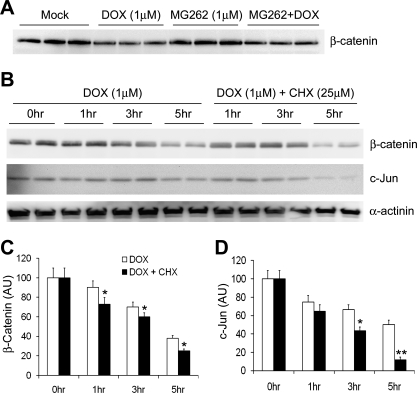
Fig. 5.
Dox enhances the degradation of endogenous proteasome substrates c-Jun and β-catenin in cultured cardiomyocytes. A: representative image of Western blot for β-catenin protein levels in NRVMs treated with Dox (1 μM) and/or MG262 (1 μM) for 16 h. Note that Dox decreased β-catenin protein levels both at baseline and in the presence of a proteasome inhibitor. B–D: CHX chase experiments for β-catenin and c-Jun proteins. Cultured adult mouse cardiomyocytes were treated with Dox (1 μM) for the indicated times in the absence or presence of CHX (25 mM) pretreatment (1 h before Dox treatment). A representative cohort of Western blot images is shown in B, and quantitative data from 4 repeats are shown in C (β-catenin) and D (c-Jun), respectively. α-Actinin (a relatively stable cytoskeletal protein) was also probed with the same membranes. To minimize the impact from potential loading variations, both β-catenin and c-Jun signals were normalized to the same lane α-actinin signal. The average value of the 0-h time point (i.e., Dox untreated) was arbitrarily set as 100 AU and was used to quantify the other time points. *P < 0.05 and **P < 0.01, Dox + CHX group compared with CHX group at the same time point.