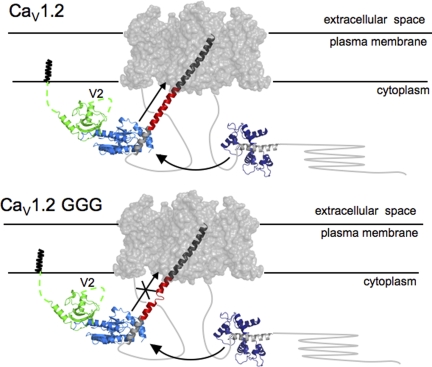
Figure 7.
Cartoon model of a CaV channel. Based on the likely gross similarly between CaV and Kv transmembrane portions, the Kv1.2 transmembrane domains (gray surface; PDB accession no. 2A79) are used to represent the CaV transmembrane domains. The IS6-AID linker (red) was modeled manually by building a helix of corresponding length between the Kv1.2 S6 helix C terminus (dark gray) and AID helix (light gray) from the CaVβ2a–AID complex (PDB accession no. 1T0J). The CaVβ2a–AID complex is shown as follows: green, SH3 domain; light blue, NK domain; light gray, AID. N-terminal CaVβ2a variable segment, V1, of unknown structure, is shown anchored to the membrane via N-terminal palmitoylation, and the V2 loop is indicated. Arrow along the IS6-AID linker indicates communication between CaVβ and the pore domain. This is lost in the multiple glycine mutants (bottom) and affects VDI, CDI, and CDF. Curved arrow between the Ca2+/CaM-IQ domain complex (PDB accession no. 2BE6), Ca2+/CaM (dark blue), and IQ helix (gray) represents the functional interaction between the C-terminal tail complex and the CaVβ2a–AID complex required for CDI and CDF. In CaV1.2 GGG (bottom), IS6-AID helix disruption blunts the influence of the Ca2+/CaM-IQ domain on the transmembrane pore.