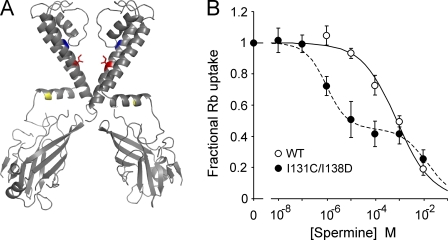
Figure 3.
The I131C/I138D mutant is more sensitive to spermine inhibition than WT. (A) Structure of two opposing subunits of KirBac1.1 highlighting the residues I131 (blue) and I138 (red) that were mutated to cysteine and asparate, respectively. R49 is also highlighted in yellow. Mutation of this residue to a cysteine renders an inactive channel that can be rescued by MTSET modification. (B) Plot of relative 86Rb+ uptake of WT and I131C/I138D in liposomes at different concentrations of externally applied spermine, normalized to uptake without spermine (n = 9 ± SEM). The I131C/I138D data are fit with the sum of two Hill functions (K1/2 = 1 µM, H = 1; K1/2 = 15 mM, H = 0.8), and the WT data with one (K1/2 = 0.8 mM, H = 0.6).