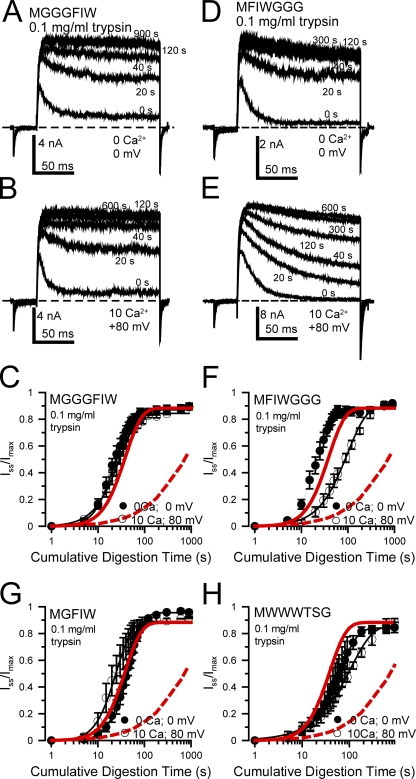
Figure 4.
Some manipulations of the β2 N terminus can alter inactivation-dependent protection against digestion, with minimal effects on rates of digestion during closed-channel conditions. (A) Traces show digestion at different times by 0.l mg/ml trypsin applied in the presence of 0 Ca2+ at 0 mV (closed-channel conditions) for the MGGGFIW construct. Displayed currents were obtained between trypsin applications with 10 µM Ca2+ as in Fig. 3. (B) Traces are shown for the MGGGFIW construct during digestion by 0.1 mg/ml trypsin in 10 µM Ca2+ at +80 mV (inactivated conditions). For this construct, at +80 mV, the steady-state non-inactivating current is ∼30–40% of the maximal current. (C) The digestion time courses for MGGGFIW are plotted for the two conditions (black symbols, closed channel; open symbols, inactivated), along with the fitted results for the wild-type β2 subunit (dashed red line, inactivated conditions [10 µM Ca2+; 0 mV]; red line, closed-channel conditions [0 Ca2+; 0 mV]). (D and E) Traces are shown for the MFIWGGG construct for digestion by 0.1 mg/ml trypsin either at 0 Ca2+, 0 mV (D) or at 10 µM Ca2+, +80 mV (E). For MFIWGGG, the steady-state non-inactivating current at +80 mV is ∼5% of the maximal current. (F) Digestion time courses are plotted for the MFIWGGG construct along with the time course for β2. (G and H) Digestion time courses are plotted for MGFIW (G) and MWWWTSG (H), both under closed-channel and inactivating conditions.