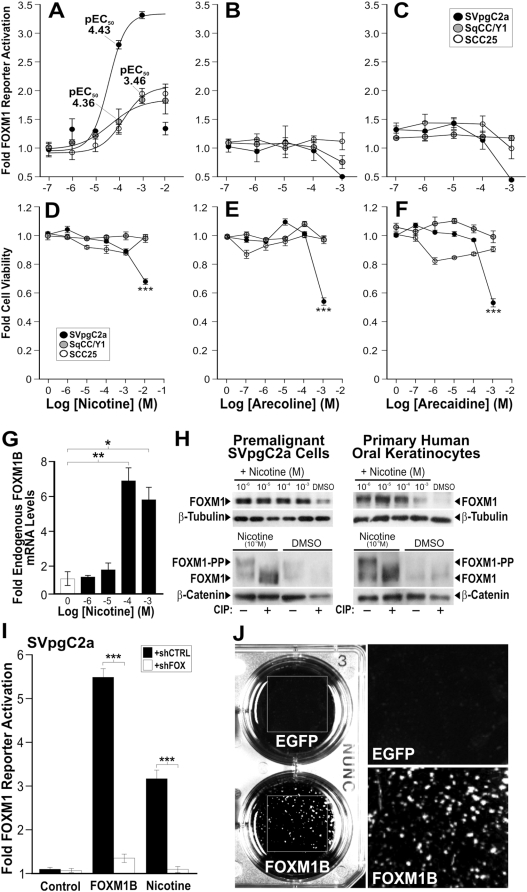
Figure 4. Nicotine activated endogenous FOXM1 transcriptional activity and promoted FOXM1B-induced malignant transformation in human oral keratinocytes.
(A–C) Dose-response curves of nicotine (A), arecoline (B) and arecaidine (C) on FOXM1 transcriptional activity in SVpgC2a, SqCC/Y1 and SCC25 cells, respectively. (D–F) Cell viability assays on SVpgC2a, SqCC/Y1 and SCC25 cells treated with the indicated doses of nicotine (D), arecoline (D) and arecaidine (F), respectively. Each data point indicates mean±SEM (n = 3). ***(P<0.001) indicates significant cell death. (G) qPCR shows that nicotine dose-dependently activated endogenous FOXM1B in SVpgC2a cells. (H) Immunoblotting shows that nicotine dose-dependently activated endogenous FOXM1 protein in both the SVpgC2a (left panel) and primary normal human oral keratinocytes (right panel) as indicated. Bottom panels showed that protein lysates were either pretreated with or without calf-intestinal phosphatase (CIP) prior to immunoblotting. Nicotine at respective optimal doses for each cell type showed reduction of the phosphorylated FOXM1 (FOXM1-PP, top bands) in CIP-treated lysates. β-Tubulin and β-catenin were used as loading control markers. (I) Reversal of nicotine-induced FOXM1 activation by RNA interference shFOX. FOXM1 transcriptional activity was assayed in SVpgC2a cells co-transfected with either shCTRL (control shRNA; solid bars) or FOXM1-specific shFOX (open bars) in either control (mock transfection), FOXM1-overexpressed or nicotine (1 mM)-treated cells, as indicated. ***(P<0.001) indicates significant repression of FOXM1 transcriptional activity over control cells. (J) Anchorage-independent cell transformation assays of EGFP or FOXM1B-overexpressing and nicotine-treated (10 mM) SVpgC2a cells.