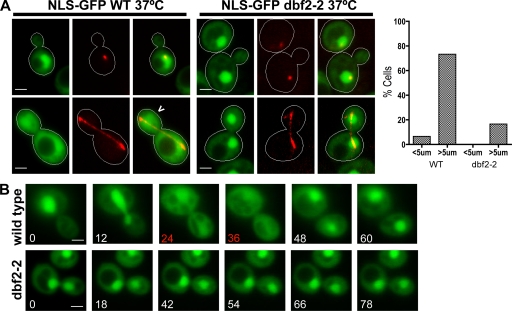
Figure 2.
Cytoplasmic localization of NLS-GFP during late mitosis is dependent on MEN. (A) Localization of NLS-GFP (green) in temperature-sensitive dbf2-2 and wild-type (WT) W303 grown at 37°C (nonpermissive). mCherry-Tub1 was imaged to identify interphase (short spindle) and late anaphase (long spindle) cells. 73% of wild-type (n = 75) cells with spindles >5 µm exhibited pancellular localization of the NLS-GFP reporter (carrot) compared with 16% of dbf2-2 (n = 50) cells, indicating that Dbf2 activity is required for inactivation of Cdc14’s C-terminal NLS during late anaphase. Only 4% of wild-type (n = 50) cells and no dbf2-2 (n = 50) cells with spindles <5 µm exhibited pancellular GFP staining. The graph shows the percentage of cells with released Cdc14 (i.e., both cytoplasmic and nuclear fluorescence) as a function of spindle length (<5 or >5 µm). (B) Wild-type and temperature-sensitive dbf2-2 yeast strains were grown at 37°C for 45 min, resuspended in 2.5% LMP agarose with 1× synthetic complete medium, and placed on slides. Slides were held at 37°C by a temperature-controlled stage for ≥30 min before imaging the cells every 6 min. Representative time-lapse series are shown for wild-type (n = 7) and dbf2-2 (n = 5) cells. The NLS-GFP reporter was released from the nucleus for approximately two time points (red) in all of the wild-type cells but exhibited persistent nuclear localization in all five dbf2-2 cells. Bars, 2 µm.