Figure 3. ND-AspRS and D-AspRS have similar but different anticodon-binding domains.
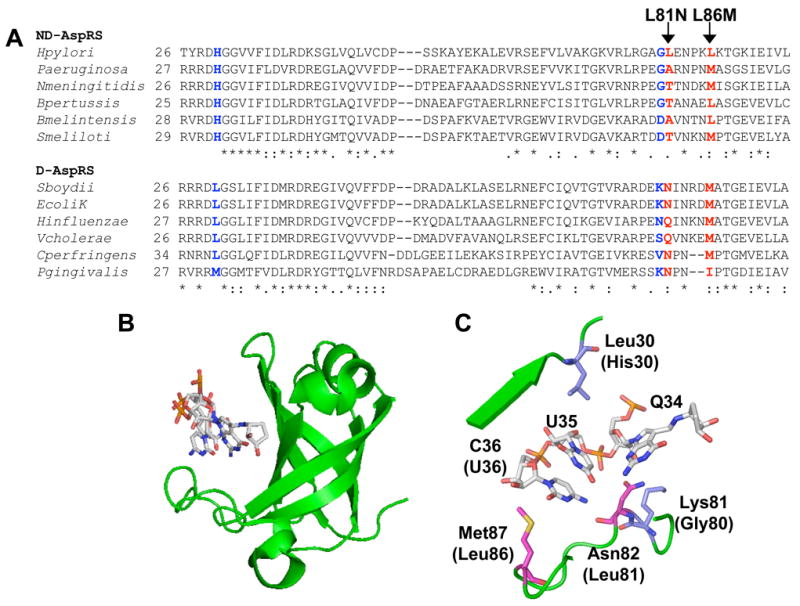
A. The anticodon-binding domains of select bacterial aspartyl-tRNA synthetase sequences were aligned (typically residues 1-100, only regions relevant to the present work are shown). Each AspRS sequence was assigned as a D-AspRS or an ND-AspRS based on genome analyses for the presence of AsnRS and Asp/Glu-Adt genes (gatCAB). Ambiguous AspRS sequences (e.g. enzymes from an organism that encodes both AsnRS and Glu-Adt) were omitted. The two residues highlighted in red were mutated (L81N and L86M) as part of the present study; those in blue were analyzed by Roy and colleagues (34). B. The anticodon-binding domain of E. coli D-AspRS complexed with fully modified E. coli tRNAAsp, showing the cleft that accommodates the anticodon trinucleotides (46). C. A close-up of this anticodon-binding domain. Residues shown in magenta were mutated as part of this study; those shown in blue were analyzed in a study by Roy and colleagues (34). See results section for details.
