Figure 4. Michaelis-Menten Kinetics for Hp Wild-type AspRS and Hp ND-AspRS Mutants.
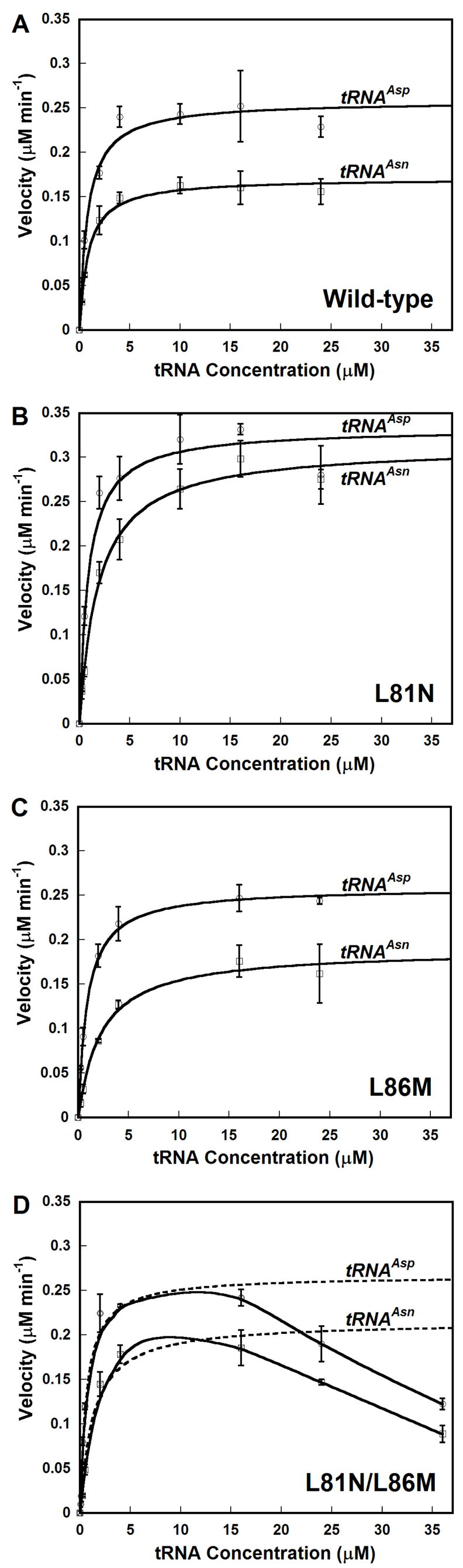
Plots for rate versus tRNA concentration are shown for each enzyme with tRNAAsp (open circles) and tRNAAsn (open squares): A. Wild-type AspRS; B. L81N; C. L86M; D. L81N/L86M. For wild-type and each single mutant, the data were directly fit to the Michaelis-Menten equation using Kaleidagraph (Synergy Software); these fits are shown as solid lines. For the L81N/L86M double mutant (Panel D), kinetic parameters were calculated using only the initial velocity values for tRNA concentrations ≤ 16 μM (broken line). At higher tRNA concentrations, significant substrate inhibition was observed (solid line); see results for detail. Error bars represent the standard deviation of experiments run in triplicate.
