Abstract
Nestin was first described as an intermediate filament protein expressed in neuroepithelial stem cells. Nestin expression has also been reported in brain tumors, schwannomas, gastrointestinal stromal tumors, and melanomas. In the pancreas, Nestin expression has been detected in exocrine and mesenchymal cells, including stellate cells, pericytes, and endothelial cells. In the present study, we examined Nestin expression in human pancreatic ductal adenocarcinoma and sought to determine its role in this malignancy. RT-PCR analysis demonstrated the presence of Nestin mRNA in all ten tested pancreatic cancer cell lines, and quantitative RT-PCR revealed that Nestin mRNA levels were highest in PANC-1 cells and lowest in PK-8 cells. Immunofluorescent analysis revealed that Nestin localized in the outer cytoplasm of PANC-1 cells. Nestin immunoreactivity was present in the cancer cells in 20 of 60 (33.3 %) cancer cases, and its expression was confirmed by in situ hybridization. Nestin expression was also increased in peripheral nerve fibers adjacent to cancer cells and in peripheral nerve fibers invaded by cancer cells. Clinicopathologically, there was a statistically significant association between Nestin expression in pancreatic cancer cells and nerve invasion (p=0.010), and the presence of cancer cells in the tumor resection margins (p=0.003). Nestin-positive cases exhibited similar survival following resection by comparison with Nestin-negative cases, irrespective of whether or not they were given adjuvant therapy. These findings indicate that Nestin expression in pancreatic cancer cells may contribute to nerve and stromal invasion in this malignancy.
Keywords: pancreatic cancer, Nestin, RT-PCR, immunohistochemistry, nerve fiber
Introduction
Pancreatic ductal adenocarcinoma (PDAC) is an aggressive malignancy with an overall 5-year survival rate that is less than 5% [1]. Clinically, this poor prognosis is due to the fact that PDAC is often diagnosed at a late stage when the cancer is no longer resectable. Moreover, patients that undergo resection frequently experience disease recurrence with a high incidence of lymph node and hepatic metastases and peritoneal dissemination [2].
Nestin, a class VI intermediate filament protein, was originally described as a neuronal stem cell/progenitor cell marker during central nervous system (CNS) development [3, 4]. Nestin is a large protein (>1600 amino acids), structurally similar to other intermediate filaments, with a highly conserved a-helical core domain of 300–330 amino-acids flanked by amino- and carboxy-terminal domains [3]. Nestin contains a short N-terminus and an unusually long C-terminus, which interacts with other cellular components and microtubules. Nestin interacts with other proteins such as vimentin or desmin, forming heterodimers and mixed polymers [5, 6].
Nestin was originally identified in stem cells that are present at the ventricular border in mammalian brains and were shown to give rise to neurons and glia [3]. Subsequently, Nestin expression was shown to be upregulated in progenitor cells in various tissues such as muscle, testis, and teeth, usually followed by decreased expression when the cells reach their differentiated state [7–9].
In adult organisms, Nestin-expressing cells are restricted to defined locations, where they may function as a cellular ‘reserve’ capable of proliferation, differentiation, and migration after re-activation [10, 11]. It has also been demonstrated that, in response to injury, the CNS, skeletal muscle, and liver can upregulate Nestin expression [12–15]. Thus, while Nestin is not detected in normal astrocytes, it is transiently present in reactive astrocytes in brain tissue [14]. Nestin is also expressed in several mature cell types such as adrenocortical cells [16] and interstitial cells of Cajal [17]. However, it remains unclear what role Nestin plays in cells that do not migrate or proliferate. Analysis of the rat Nestin promoter in transgenic mice indicates that the region upstream of the first exon does not contain any identifiable regulatory elements [18]. Nonetheless, Nestin expression in muscle precursors and neuroepithelial stem cells in the CNS is independently regulated by temporally and spatially restricted enhancer elements in the first and second introns.
In the adult pancreas, Nestin-positive cells were initially described as a specific subpopulation of cells located in the endocrine islets and having a possible stem-cell function [19]. Immunohistochemical studies indicated that Nestin is present in mesenchymal and endothelial cells, whereas lineage-tracing experiments indicated that mostly exocrine cells are derived from Nestin-expressing progenitor cells [20]. In vivo, Nestin is not present in endocrine cells during either embryogenesis or adulthood, underscoring the conclusion that endocrine cells do not derive from Nestin-expressing progenitors. Moreover, it has been recently demonstrated that activation of oncogenic Kras in the Nestin cell lineage is sufficient for the initiation of premalignant pancreatic intraepithelial neoplasia (PanIN) lesions in the pancreas [21].
Nestin expression was originally reported in nervous system tumors such as astrocytomas, ependymomas, oligodendrogliomas, glioblastomas, schwannomas, and primitive neuroectodermal tumors [22–26]. It was also shown to be expressed in gastrointestinal stromal tumors and in melanomas, where its expression has been correlated with advanced disease state and metastasis [17, 27, 28]. Very recently, several studies reported that Nestin may also be expressed in epithelial-derived tumors. Thus, Nestin has been reported in carcinomas of the breast and prostate [29, 30], in cultured pancreatic cancer cell lines [21], and in PDACs [31, 32].
In the present study, we sought to further elucidate the potential role of Nestin in PDAC. We now report that Nestin expression in PDAC cells correlates with the presence of nerve invasion and positive surgical margins.
Materials and methods
Materials
The following were purchased: pGEM-T vectors from Promega Biotech. (Madison, WI, USA); a digoxigenin (DIG) nucleic acid detection kit, a DIG RNA labeling kit, Transcriptor first strand cDNA synthesis kit and LightCycler-FastStartDNA Master SYBR Green I system from Roche Diagnostics (Mannheim, Germany); yeast tRNA from Gibco BRL (Gaithersburg, MD, USA); Tween 20, glycine, and formamide from Wako Pure Chemical Industries, Ltd. (Osaka, Japan); a Takara RNA PCR kit (AMV) Ver. 3.0 from Takara Biotech. (Tokyo, Japan); RNeasy mini kit from QIAGEN GmbH (Hilden, Germany); rabbit polyclonal anti-S-100 antibodies from DakoPatts (Glostrup, Denmark); mouse monoclonal anti-Nestin antibodies from R&D Systems Inc. (Westerville, OH, USA); a Histofine Simple Stain Max PO (M) or (R) kit from Nichirei Biosciences, Inc. (Tokyo, Japan); Human Tissue Microarray 1 and Human Digestive Tissue Sets from Novagen (Darmstadt, Germany); Fluorescein 5-isothiocyanate (FITC)-conjugated anti-mouse IgG and Vectashield mounting medium containing 4′, 6-diamidino-2-phenylindole dihydrochloride (DAPI) from Vector Laboratories, Inc. (Burlingame, CA, USA); Silane coated slides from Muto Chemical Co. (Tokyo, Japan); All other chemicals and reagents were purchased from Sigma Chemical Corp. (St. Louis, MO, USA).
Patients and tissues
Tissues from 60 patients with invasive PDAC were obtained for this study. These patients received treatment at Nippon Medical School Hospital (Bunkyo-ku, Tokyo, Japan) from 1995 to 2005. None of the patients received preoperative chemotherapy or radiotherapy. The patients consisted of 35 males and 25 females, whose median age was 64.6 years (range, 35–84 years). The clinicopathologic stage was determined according to the TNM classification system of the International Union Against Cancer (UICC) and additionally characterized according to the Japan Pancreas Society classification (Table 1). Thirty patients did not receive any postoperative chemotherapy, whereas 30 patients received adjuvant chemotherapy following surgery. Fourteen patients received Uracil/Tegafur and 18 patients received gemcitabine. The median follow-up period was 14.7 months. Paraffin-embedded specimens were prepared for immunohistochemical analysis as described previously [33]. This study was carried out in accordance with the principles embodied in the Declaration of Helsinki, 1975 and informed consent for the usage of pancreatic tissues was obtained from each patient. Normal pancreatic tissues were obtained from Human Digestive Tissue Sets and Human Tissue Microarray 1.
Table 1.
Correlation of clinicopathological features and nestin expression in pancreatic cancers.
| Variables | No. | Nestin (%) | P |
|---|---|---|---|
| Gender | |||
| Male | 35 | 13 (37) | |
| Female | 25 | 7 (28) | NS |
| Age | |||
| <65 | 27 | 9 (33) | |
| ≧65 | 33 | 11 (33) | NS |
| UICC classification | |||
| T-primary tumor | |||
| T1 | 4 | 0 (0) | |
| T2 | 2 | 1 (50) | |
| T3 | 30 | 11 (37) | |
| T4 | 24 | 8 (33) | NS |
| N-Regional lymph nodes | |||
| N0 | 20 | 6 (30) | |
| N1 | 40 | 14 (35) | NS |
| M-Distant metastasis | |||
| M0 | 58 | 20 (34) | |
| M1 | 2 | 0 (0) | NS |
| G-Histological grading | |||
| G1 | 33 | 10 (30) | |
| G2 | 23 | 8 (33) | |
| G3 | 4 | 2 (50) | |
| G4 | 0 | NS | |
| Stage | |||
| I or II | 3 | 0 (0) | |
| III or IV | 57 | 20 (35) | NS |
| Other tumor characteristics | |||
| Nerve invasion (intrapancreatic) | |||
| negative | 11 | 0 (0) | |
| positive | 49 | 20 (41) | p=0.010 |
| DPM (dissected peripancreatic tissue margin) | |||
| negative | 34 | 6 (18) | |
| positive | 26 | 14 (54) | p=0.003 |
Pancreatic cancer cell lines
PANC-1, MIA PaCa-2, KLM-1, and PK-1, 8, 9, 45H, 45P and 59, PDAC cell lines were obtained from the Cell Resource Center for Biomedical Research, Institute of Development, Aging and Cancer, Tohoku University (Sendai, Japan), and Capan-1 was purchased from American Type Culture Collection. The cells were grown in the RPMI 1640 medium containing 10% heat-inactivated fetal bovine serum (FBS), 200 U/ml penicillin and 200 μg/ml kanamycin at 37 °C under a humidified 5% CO2 atmosphere. Capan-1 was incubated in same medium with 15% FBS.
Reverse transcription-polymerase chain reaction (RT-PCR)
Total RNA was extracted from pancreatic cancer cell lines using the RNeasy mini kit according to the manufacturer’s protocol. Then, cDNA synthesis and PCR were performed using the Takara RNA PCR kit. The primer pair used for Nestin corresponded to nucleotides (nts) 1019–1044 (5′-GGA-TCC-GAG-GTG-GCC-ACG-TAC-AGG-AC-3′) and nts 1228–1253 (5′-GAA-TTC-GAA-AGG-CTG-GCA-CAG-GTG-TC-3′) (235bp, accession No. NM_006617). β-actin mRNA, as the positive control, was amplified using the following primer pairs: nts 254–273 (5′-AAG-AGA-GGC-ATC-CTC-ACC-CT-3′) and nts 452–471 (5′-TAC-ATG-GCT-GGG-GTG-TTG-AA-3′) (218 bp, accession No. NM_001101). Authenticity of the PCR product was confirmed by sequencing. Total RNA not subjected to reverse transcription was used as the negative control.
Quantitative RT-PCR
One microgram of total RNA sample was used for RT with the Transcriptor first strand cDNA synthesis kit following the manufacture’s protocol. Quantitative RT-PCR (qRT-PCR) was performed using a LightCycler-FastStartDNA Master SYBR Green I system. The primer pairs used for Nestin corresponded to nts 1204–1255 (5′-CTC-ACC-CTT-GCC-TGC-TAC-CCT-T-3′) and nts 1334–1358 (5′-CCT-GGG-CTC-TGA-TCT-CTG-CAT-CTA-C-3′) of the human Nestin cDNA (155bp, accession No. NM_006617). β-actin mRNA was amplified using the following primer pairs: nts 331–353 (5′-GCA–CCA-CAC-CTT-CTA-CAA-TGA-GC-3′) and nts 472–493 (5′-TAG-CAC-AGC-CTG-GAT-AGC-AAC-G-3′) (163 bp, accession No. NM_01101). Twenty microliters of PCR reaction mixture containing 2 μl of template cDNA, 3 mM MgCl2, 0.5 μM of primers, and LightCycler-FastStartDNA Master SYBR Green I mix was applied into the capillary tube. qRT-PCR was carried out in a LightCycler, and the PCR product was analyzed by Light Cycler Data Analysis software Ver. 3.5 (Roche Diagnostics GmbH). To confirm amplification specificity, PCR products were subjected to a melting curve analysis. Results were expressed as Nestin/β-actin, as an internal standard concentration ratio. The optimized program involved denaturation at 95 °C for 10 minutes, followed by 45 cycles of amplification: (at 95 °C for 10 seconds, at 58 °C for 10 seconds, at 72 °C for 12 seconds) for Nestin; by 40 cycles of amplification: (at 95 °C for 10 seconds, at 64 °C for 10 seconds, at 72 °C for 7 seconds) for β-actin.
Immunofluorescence staining and confocal laser microscopy
A highly specific anti-Nestin monoclonal antibody was used to perform immunofluorescence staining. PANC-1 cells were incubated for 1 hour at 23 °C with the anti-Nestin antibody (1:100) using phosphate-buffered saline (PBS) containing 1% bovine serum albumin (BSA). Incubation with PBS containing 1% BSA served as a negative control. The cells were washed with PBS and then incubated with FITC-conjugated anti-mouse IgG. One hour after incubation, the cells were washed with PBS and then mounted with Vectashield mounting medium containing DAPI. Fluorescent images were acquired using a confocal laser scanning microscope Digital Eclipse TE 2000-E (Nikon Insteck Co., Ltd. Tokyo, Japan) and a 100X immersion lens (Nikon Palm Apo VC) with blue diode and argon lasers and were analyzed using the confocal microscope Digital Eclipse C1 control software EZ-C1 (version 2.30) (Nikon Insteck). The excitation wavelength for FITC was 488 nm, and emission was selected and recorded using a 500- to 530-nm band-pass filter. In addition, the excitation wavelength for DAPI was 405 nm, and emission was selected and recorded using a 432- to 446-nm band-pass filter.
Immunohistochemistry
Paraffin-embedded tissue sections (3.5 μm) were subjected to immunostaining using Histofine Simple Stain Max PO (M) or (R) kits. After deparaffinization, endogenous peroxidase activity was blocked by incubating for 30 minutes with 0.3% hydrogen peroxide in methanol. Sections were then incubated for 16 hours at 4 °C in the absence (negative controls) or presence of monoclonal anti-Nestin (1:100 dilution) or anti-S-100 (1:1000 dilution) antibodies. Bound antibodies were detected with Histofine Kits, using diaminobenzidine-tetrahydrochloride as the substrate. All sections were counterstained with Mayer’s hematoxylin. Immunohistochemical results for Nestin were evaluated as follows: when staining was noted in the cytoplasm and/or membrane of more than 10% of the tumor cells, regardless of the intensity of staining, the cells were designated as positive. Two investigators (M. Kawamoto. and T. Ishiwata) separately evaluated all the specimens in a blinded manner. We chose the 10% cutoff because Nestin was completely absent in the normal human exocrine pancreas, which raised the possibility that even a relatively small number of Nestin-positive cancer cells (at least 10% of the cells) may play role in PDAC.
In situ hybridization analysis
A 235-bp BamHI-EcoRI cDNA fragment, corresponding to nucleotides 1045–1227 of the human Nestin cDNA sequence, was subcloned into the pGEM-T vector and the results were confirmed by sequencing. Probes were labeled with digoxigenin-UTP using SP6 or T7 RNA polymerase and the DIG RNA-labeling kit. In situ hybridization was carried out as previously reported [34]. Tissue sections were deparaffinized and incubated at RT for 20 minutes with 0.2 M HCl and then at 37 °C for 15 minutes with 100 μg/mL proteinase K. The sections were postfixed for 5 minutes in PBS containing 4% paraformaldehyde, and incubated twice for 15 minutes each in PBS containing 2 mg/mL glycine and once in 2X standard saline citrate (SSC) containing 50% formamide for 1 hour before the initiation of the hybridization reaction. Hybridization was carried out in a moist chamber for 16 hours at 42 °C. The sections were then washed sequentially with 2X SSC for 20 minutes at 42 °C, and with 0.2X SSC for 20 minutes at 42 °C. For immunological detection, the DIG nucleic acid detection kit was used. The sections were washed briefly with buffer 1 (100 mM Tris HCl and 150 mM NaCl, pH 7.5), incubated with 1% (w/v) blocking reagents in buffer 1 for 1 hour at room temperature, and thereafter incubated with alkaline phosphatase-conjugated polyclonal sheep anti-DIG Fab fragment at 1:2000 dilution for 1 hour at RT. The sections were then washed twice for 15 minutes at RT with buffer 1 containing 0.2% Tween 20, equilibrated in buffer 3 (100 mM Tris HCl, 100 mM NaCl and 50 mM MgCl2, pH 9.5) for 2 minutes, and incubated in the staining solution containing nitroblue tetrazolium and X-phosphate in a dark box for 2 to 3 hours. After the reaction was stopped with a Tris-EDTA acid buffer (10 mM Tris-HCl and 1 mM EDTA, pH 8.0), the sections were mounted in an aqueous mounting medium.
Statistical analysis
Whenever indicated, the chi-square test and Fisher’s exact test were used to analyze the correlation between Nestin expression and clinicopathologic features. Cumulative survival rates were calculated by the Kaplan-Meier method, and the significance of differences in survival rate was analyzed by the log-rank test; P < 0.05 was considered significant in all analyses. Computations were performed using the StatView J version 4.5 software package (SAS Institute, Inc., Cary, NC, USA).
Results
RT-PCR analysis of Nestin expression in pancreatic cancer cell lines
Nestin mRNA expression was examined in pancreatic cancer cell lines, including PANC-1, MIA PaCa-2, Capan-1, KLM-1 and PK-1, 8, 9, 45H, 45P and 59. A 235-bp band corresponding to Nestin mRNA was detected in all pancreatic cancer cell lines (Fig. 1, upper panel). A 218-bp band corresponding to β-actin mRNA was used as a loading control and was detected in all cancer cell lines (Fig. 1, lower panel).
Figure 1. RT-PCR analysis of Nestin mRNA in pancreatic cancer cell lines.

Total RNA was extracted from PANC-1, MIA PaCa-2, Capan-1, KLM-1 and PK-1, 8, 9, 45H, 45P and 59 and used to perform cDNA synthesis and PCR analysis. Nestin mRNA (235-bp) was detected in all cell lines (upper panel). β-actin mRNA (218-bp) served as a loading control (lower panel).
Quantitative RT-PCR analysis of Nestin mRNA levels
To determine the relative expression levels of Nestin mRNA in pancreatic cancer cell lines, qRT-PCR was performed next. Nestin mRNA levels were highest in PANC-1 cells, and lowest in PK-8 cells (Fig. 2). In PANC-1 cells, Nestin mRNA levels were 137-fold higher than in PK-8 cells.
Figure 2. Quantitative RT-PCR analysis of Nestin mRNA levels.
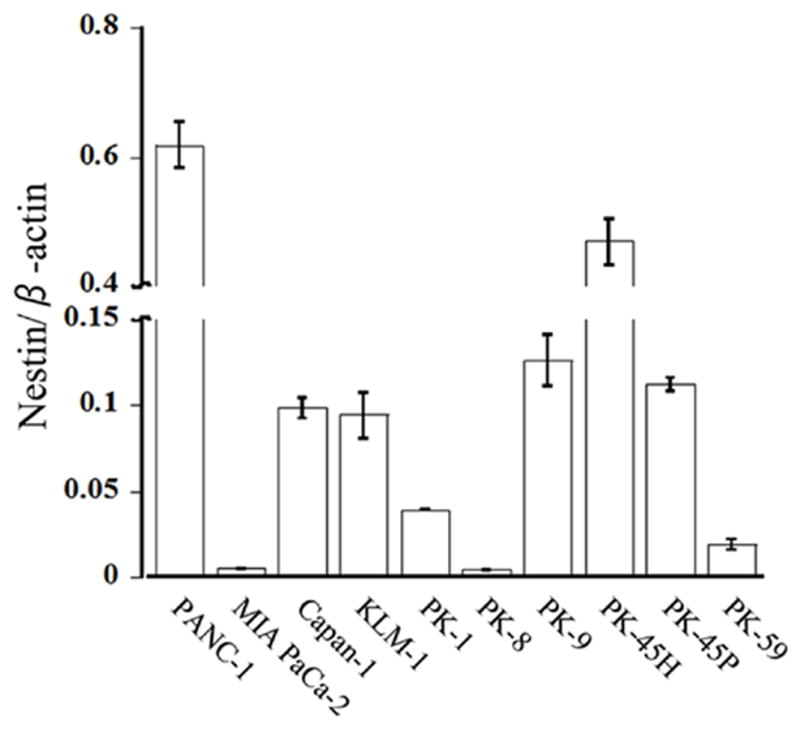
Nestin mRNA levels were highest in PANC-1 cells, and lowest in PK-8 cells. In PANC-1 cells, Nestin mRNA levels were approximately 137-fold higher than in PK-8 cells.
Immunofluorescence staining and confocal laser microscopy
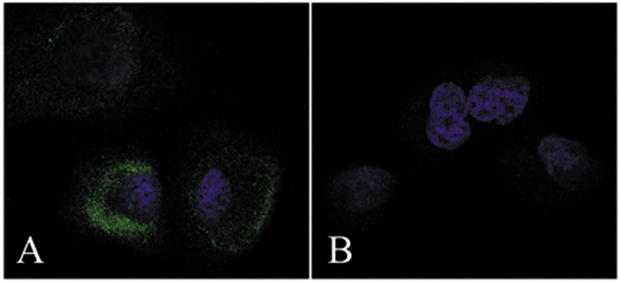
Immunofluorescence staining using confocal laser microscopy was performed next, in order to assess Nestin protein levels in PANC-1 cells. This analysis revealed that Nestin was present in the cytoplasm of PANC-1 cells, with some cells exhibiting a strong Nestin signal at the periphery of the cytoplasm (Fig. 3A). Nestin was not detected when incubations were performed in the absence of the anti-Nestin antibody (Fig. 3B).
Figure 3. Immunofluorescent analysis of Nestin expression in PANC-1 cells.
Nestin immunoreactivity was detected in the cytoplasm of cancer cells (A). In some cases, a strong Nestin signal was detected in the periphery of the cytoplasm. Nestin signals were not detected in negative control cells without anti-nestin antibody (B).
Immunohistochemical and in situ hybridization analysis of Nestin expression in the normal pancreas and in pancreatic cancer tissues
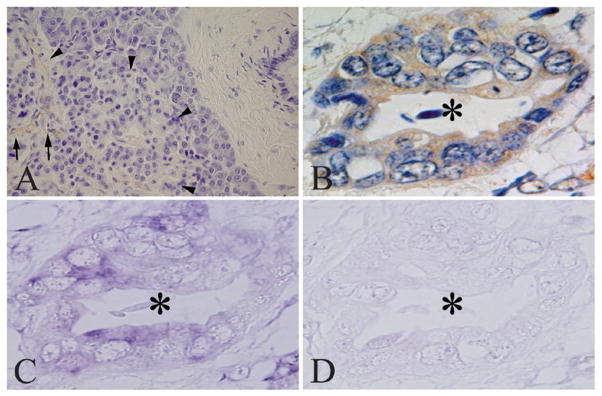
Immunohistochemical and in situ hybridization studies were carried out next, in order to characterize Nestin expression in the normal pancreas and in PDAC. Nestin was localized in a few capillary endothelial cells within the islets in normal pancreatic tissues (Fig. 4A, arrows), but was not detected in acinar, ductal, or islet cells (arrowheads). Nestin immunoreactivity, defined as being present in at least 10% of the cancer cells, was detected in the cytoplasm of cancer cells in 20 of 60 PDAC samples (Fig. 4B, asterisk). Interestingly, most of the cancer samples that were characterized as having less than 10% Nestin-positive cells (40 cases) were completely negative for Nestin. In situ hybridization analysis revealed that Nestin mRNA was expressed in the cancer cells in serial tissue sections (Fig. 4C, asterisk), often, but not always, in the same cells that were positive for Nestin immunoreactivity. By contrast, in situ hybridization using a sense probe did not produce any signal (Fig. 4D, asterisk). In situ hybridization was next carried out on serial sections from four additional cases that were Nestin positive by immunostaining. In all four cases, the in situ hybridization signal corresponding to Nestin mRNA was present in nearly all the cancer cells that exhibited Nestin immunoreactivity (Fig. 5A–H), underscoring the validity of the results obtained by immunohistochemistry. Clinicopathologically, there was a significant correlation between Nestin expression in the cancer cells, nerve invasion, and cancer-positive surgical margins (P=0.010 and 0.003, respectively; Table 1).
Figure 4. Nestin expression in the normal pancreas and in pancreatic cancer.
Nestin was expressed in a few capillary endothelial cells (arrows) in the endocrine islets (arrowheads) in normal pancreatic tissues (A). Nestin and its mRNA were expressed in cytoplasm of pancreatic cancer cells (B and C, asterisk) in the PDAC samples. Sense probes did not yield any positive signals in the cancer cells (D asterisk). A and B, immunohistochemistry: C and D, in situ hybridization analysis.
Original magnification: A×200; B, C and D×600
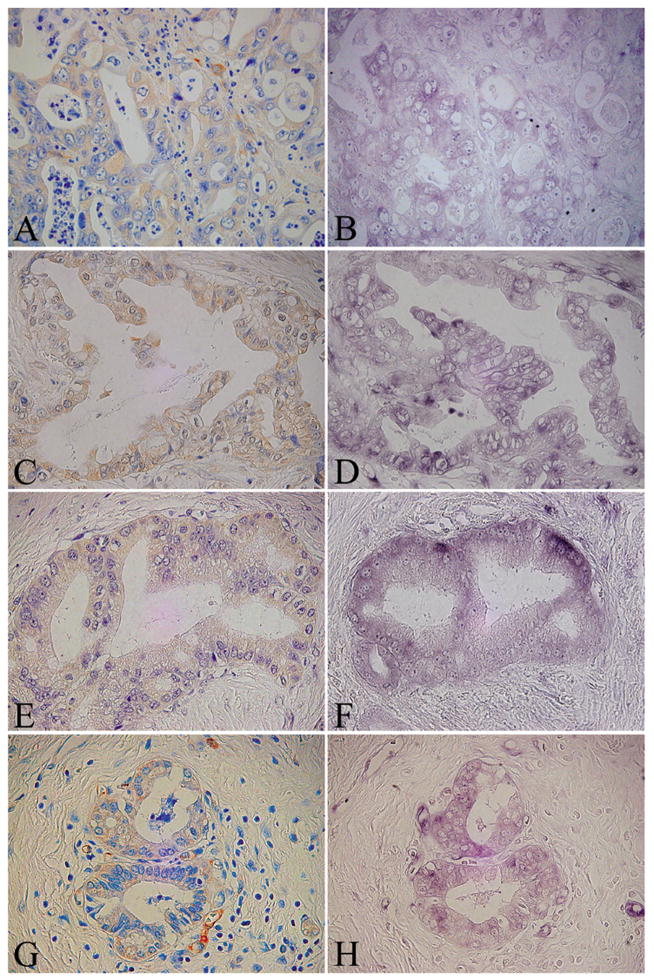
Figure 5. Characteristic expression pattern of Nestin and its mRNA in pancreatic cancer cases.
Serial tissue sections of four different pancreatic cancer cases were subjected to immunohistochemistry and in situ hybridization analysis. A and B: Case 3, C and D: Case 10, E and F: Case 25, G and H: Case 35. A, C, E and G, immunohistochemistry: B, D, F and H, in situ hybridization analysis.
Original magnification: A-H×600
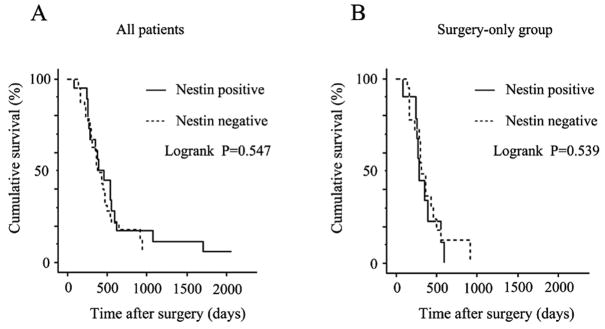
Cumulative Kaplan-Meier survival curve
The overall 2-year survival rate of all 60 cases of PDAC was 16.7 %. The overall survival rates of the Nestin-positive group and Nestin-negative group were not significantly different (P=0.547, Fig. 6A). Moreover, in the group that underwent surgical resection without receiving adjuvant therapy, the survival duration of the Nestin-positive group was not significantly different from the Nestin-negative group (P = 0.539, Fig. 6B).
Figure 6. Cumulative Kaplan-Meier survival curves.
Cumulative survival plots of the post-operative survival periods are shown for all patients (A) and for patients who underwent tumor resection but did not receive adjuvant chemotherapy (B). The survival of patients with Nestin-positive or negative tumors was similar, irrespective of whether all the patients were evaluated (p=0.547, A) or the surgery-only group was evaluated (P=0.539, B).
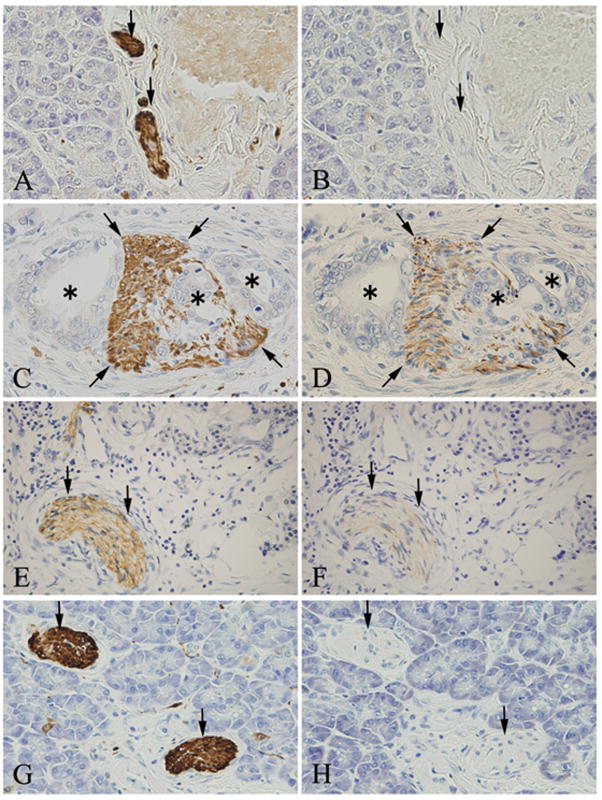
Immunohistochemical analysis of Nestin in nerve fibers
S-100 protein was detected in all nerve fibers in pancreatic tissues (Figs. 7A, C, E, and G, arrows). In the normal pancreas, peripheral nerve fibers did not exhibit Nestin immunoreactivity (Fig. 7B). By contrast, in the PDAC samples, nerve fibers that were either invaded by cancer cells (Fig. 7D) or were located in the chronic pancreatitis (CP)-like lesions adjacent to the cancer cells (Fig. 7F), were Nestin-positive, whereas nerve fibers that were located at some distance from either the cancer cells or the CP-like regions were Nestin-negative (Fig. 7H).
Figure 7. Immunohistochemical analysis of Nestin in nerve fibers.
Peripheral nerve fibers were stained with anti-S-100 protein (A, C, E and G, arrows). Nestin was not present in the nerve fibers in normal pancreatic tissues (B, arrows), but was present in nerve fibers (D, arrows) invaded by cancer cells (D, asterisks). Nestin was also present in the nerve fibers in chronic pancreatitis (CP)-like lesions that were adjacent to the cancer cells (F, arrows), but not when such lesions were distant from the cancer cells (H, arrows).
Original magnification: A-H×400
Discussion
Nestin expression in malignant tumors was previously reported in melanoma, anaplastic astrocytoma, anaplastic oligodendroglioma, leiomyosarcoma, gastrointestinal tumor, glioblastoma, anaplastic ependymoma, rhabdomyosarcoma, and malignant sheath tumor [22–28, 35]. Very recently, however, Nestin expression has also been reported in breast and prostate cancer [29, 30] as well as in pancreatic cancer [31, 32]. In the present study, Nestin mRNA and protein were expressed in all tested pancreatic cancer cell lines. Analysis by qRT-PCR revealed that Nestin mRNA expression levels varied across the different pancreatic cancer cell lines. Thus, Nestin mRNA levels in MIA PaCa-2 and PK-8 cells were markedly lower than in the other cancer cell lines. While the reasons for these differences are not readily evident, they reflect the heterogeneous nature of Nestin expression in pancreatic cancer cells within the PDAC tumor samples.
In agreement with previous reports [19, 20], Nestin immunoreactivity in the normal pancreas was present in a few capillary endothelial cells but was not detected in acinar, islet, or ductal cells. By contrast, Nestin immunoreactivity was present in cytoplasm of cancer cells in 20 of 60 PDAC tissues. Our findings in this regard are thus in general agreement with the findings by Yang, et al., who reported that 46% of 22 PDAC samples were positive for Nestin [32]. By contrast, in a study by Ohike et al., only 5% of the PDAC samples were Nestin positive [31]. While the differences between the studies could be due to differences between the anti-Nestin antibodies that were used by the investigators, they could also be due to differences in histological techniques. Irrespective of the reasons for these differences, our findings were confirmed by demonstrating nearly complete concordance between the immunostaining and in situ hybridization signals in serial sections from 5 of 5 tumor samples.
In the present study we also determined that there was a significant correlation between Nestin expression in the cancer cells, nerve invasion, and cancer-positive surgical margins. We hypothesize, therefore, that Nestin expression in PDAC may reflect the migration and invasive potential of the cancer cells. In support of this possibility, Nestin was reported to be abundant in the infiltrating component of melanomas [28], and to correlate with prostate cancer cell invasive potential [30]. These observations suggest that Nestin may also play an important role in the transformation from a benign to a malignant state in these tumors. However, we did not find any significant correlations between higher Nestin levels and certain clinicopathological findings, such as prognosis or survival, underscoring the multiple defects that exist in PDAC that serve to contribute to its biological aggressiveness. For example, the concomitant overexpression of the EGF receptor (EGFR) and transforming growth factor-alpha (TGF-α) is associated with disease progression, whereas the absence of EGFR and TGF-α is associated with an improved prognosis in PDAC [36]. Nonetheless, Nestin immunoreactivity was present in only one of six T1 or T2 cases (16.7%), raising the possibility that its expression in the cancer cells may correlate with extra-pancreatic invasion. Therefore, additional studies are needed to clarify the role of Nestin in invasion and migration.
Nestin was also present in peripheral nerve fibers in CP-like lesions that were adjacent to the cancer cells. Many cytokines and growth factors and their receptors are expressed in these CP-like lesions [37–39]. Moreover, Nestin expression is re-induced in the adult during pathological situations, such as during the formation of glial scars after CNS injury and during regeneration of injured muscle tissue [12, 14, 15]. Expression of the Nestin gene is driven by a minimal promoter, present between residues −11 and +183 in the 5-non-coding region [18]. The promoter contains two adjacent Sp1-binding sites necessary for promoter activity, but lacks a functional TATA box. Thus, Nestin re-activation following injury may be due to several independent molecular mechanisms, including extracellular factors, cell-cell interactions, transcriptional regulation, intermediate filament remodeling, and recruitment of stem or progenitor cells that express Nestin. Taken together, our findings suggest that Nestin may play an important role in nerve and stromal invasion in PDAC.
Acknowledgments
We express our appreciation to Mr. Takenori Fujii and Kiyoshi Teduka and Ms. Yoko Kawamoto, Taeko Suzuki and Kiyoko Kawahara (Department of Integrative Pathology, Nippon Medical School) for their excellent technical assistance. We also thank Dr. Yuichi Sugisaki (Division of Surgical Pathology, Nippon Medical School Hospital) for preparing tissue blocks. This work was supported by a Grant in Aid for Scientific Research (C, No. 17591445, 18591526) from the Japan Society for the Promotion of Science, and, in part, by U.S. Public Health Service Grants CA-75059 and CA-101306 to M. Korc.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ. Cancer statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Warshaw AL, Fernandez-del Castillo C. Pancreatic carcinoma. N Engl J Med. 1992;326:455–65. doi: 10.1056/NEJM199202133260706. [DOI] [PubMed] [Google Scholar]
- 3.Lendahl U, Zimmerman LB, McKay RD. CNS stem cells express a new class of intermediate filament protein. Cell. 1990;60:585–95. doi: 10.1016/0092-8674(90)90662-x. [DOI] [PubMed] [Google Scholar]
- 4.Yang J, Cheng L, Yan Y, Bian W, Tomooka Y, Shiurba R, Jing N. Mouse nestin cDNA cloning and protein expression in the cytoskeleton of transfected cells. Biochim Biophys Acta. 2001;1520:251–4. doi: 10.1016/s0167-4781(01)00275-5. [DOI] [PubMed] [Google Scholar]
- 5.Marvin MJ, Dahlstrand J, Lendahl U, McKay RD. A rod end deletion in the intermediate filament protein nestin alters its subcellular localization in neuroepithelial cells of transgenic mice. J Cell Sci. 1998;111(Pt 14):1951–61. doi: 10.1242/jcs.111.14.1951. [DOI] [PubMed] [Google Scholar]
- 6.Sjoberg G, Jiang WQ, Ringertz NR, Lendahl U, Sejersen T. Colocalization of nestin and vimentin/desmin in skeletal muscle cells demonstrated by three-dimensional fluorescence digital imaging microscopy. Exp Cell Res. 1994;214:447–58. doi: 10.1006/excr.1994.1281. [DOI] [PubMed] [Google Scholar]
- 7.Sejersen T, Lendahl U. Transient expression of the intermediate filament nestin during skeletal muscle development. J Cell Sci. 1993;106(Pt 4):1291–300. doi: 10.1242/jcs.106.4.1291. [DOI] [PubMed] [Google Scholar]
- 8.Frojdman K, Pelliniemi LJ, Lendahl U, Virtanen I, Eriksson JE. The intermediate filament protein nestin occurs transiently in differentiating testis of rat and mouse. Differentiation. 1997;61:243–9. doi: 10.1046/j.1432-0436.1997.6140243.x. [DOI] [PubMed] [Google Scholar]
- 9.Terling C, Rass A, Mitsiadis TA, Fried K, Lendahl U, Wroblewski J. Expression of the intermediate filament nestin during rodent tooth development. Int J Dev Biol. 1995;39:947–56. [PubMed] [Google Scholar]
- 10.Wiese C, Rolletschek A, Kania G, Blyszczuk P, Tarasov KV, Tarasova Y, Wersto RP, Boheler KR, Wobus AM. Nestin expression--a property of multi-lineage progenitor cells? Cell Mol Life Sci. 2004;61:2510–22. doi: 10.1007/s00018-004-4144-6. [DOI] [PubMed] [Google Scholar]
- 11.Teranishi N, Naito Z, Ishiwata T, Tanaka N, Furukawa K, Seya T, Shinji S, Tajiri T. Identification of neovasculature using nestin in colorectal cancer. Int J Oncol. 2007;30:593–603. [PubMed] [Google Scholar]
- 12.Aarimaa V, Kaariainen M, Vaittinen S, Tanner J, Jarvinen T, Best T, Kalimo H. Restoration of myofiber continuity after transection injury in the rat soleus. Neuromuscul Disord. 2004;14:421–8. doi: 10.1016/j.nmd.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 13.Niki T, Pekny M, Hellemans K, Bleser PD, Berg KV, Vaeyens F, Quartier E, Schuit F, Geerts A. Class VI intermediate filament protein nestin is induced during activation of rat hepatic stellate cells. Hepatology. 1999;29:520–7. doi: 10.1002/hep.510290232. [DOI] [PubMed] [Google Scholar]
- 14.Lin RC, Matesic DF, Marvin M, McKay RD, Brustle O. Re-expression of the intermediate filament nestin in reactive astrocytes. Neurobiol Dis. 1995;2:79–85. doi: 10.1006/nbdi.1995.0008. [DOI] [PubMed] [Google Scholar]
- 15.Holmin S, Almqvist P, Lendahl U, Mathiesen T. Adult nestin-expressing subependymal cells differentiate to astrocytes in response to brain injury. Eur J Neurosci. 1997;9:65–75. doi: 10.1111/j.1460-9568.1997.tb01354.x. [DOI] [PubMed] [Google Scholar]
- 16.Bertelli E, Regoli M, Lucattelli M, Bastianini A, Fonzi L. Nestin expression in rat adrenal gland. Histochem Cell Biol. 2002;117:371–7. doi: 10.1007/s00418-002-0386-2. [DOI] [PubMed] [Google Scholar]
- 17.Tsujimura T, Makiishi-Shimobayashi C, Lundkvist J, Lendahl U, Nakasho K, Sugihara A, Iwasaki T, Mano M, Yamada N, Yamashita K, Toyosaka A, Terada N. Expression of the intermediate filament nestin in gastrointestinal stromal tumors and interstitial cells of Cajal. Am J Pathol. 2001;158:817–23. doi: 10.1016/S0002-9440(10)64029-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cheng L, Jin Z, Liu L, Yan Y, Li T, Zhu X, Jing N. Characterization and promoter analysis of the mouse nestin gene. FEBS Lett. 2004;565:195–202. doi: 10.1016/j.febslet.2004.03.097. [DOI] [PubMed] [Google Scholar]
- 19.Hunziker E, Stein M. Nestin-expressing cells in the pancreatic islets of Langerhans. Biochem Biophys Res Commun. 2000;271:116–9. doi: 10.1006/bbrc.2000.2611. [DOI] [PubMed] [Google Scholar]
- 20.Ishiwata T, Kudo M, Onda M, Fujii T, Teduka K, Suzuki T, Korc M, Naito Z. Defined localization of nestin-expressing cells in L-arginine-induced acute pancreatitis. Pancreas. 2006;32:360–8. doi: 10.1097/01.mpa.0000220860.01120.21. [DOI] [PubMed] [Google Scholar]
- 21.Carriere C, Seeley ES, Goetze T, Longnecker DS, Korc M. The Nestin progenitor lineage is the compartment of origin for pancreatic intraepithelial neoplasia. Proc Natl Acad Sci U S A. 2007;104:4437–42. doi: 10.1073/pnas.0701117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Almqvist PM, Mah R, Lendahl U, Jacobsson B, Hendson G. Immunohistochemical detection of nestin in pediatric brain tumors. J Histochem Cytochem. 2002;50:147–58. doi: 10.1177/002215540205000203. [DOI] [PubMed] [Google Scholar]
- 23.Duggal N, Hammond RR. Nestin expression in ganglioglioma. Exp Neurol. 2002;174:89–95. doi: 10.1006/exnr.2001.7838. [DOI] [PubMed] [Google Scholar]
- 24.Schiffer D, Manazza A, Tamagno I. Nestin expression in neuroepithelial tumors. Neurosci Lett. 2006;400:80–5. doi: 10.1016/j.neulet.2006.02.034. [DOI] [PubMed] [Google Scholar]
- 25.Dahlstrand J, Collins VP, Lendahl U. Expression of the class VI intermediate filament nestin in human central nervous system tumors. Cancer Res. 1992;52:5334–41. [PubMed] [Google Scholar]
- 26.Sugawara K, Kurihara H, Negishi M, Saito N, Nakazato Y, Sasaki T, Takeuchi T. Nestin as a marker for proliferative endothelium in gliomas. Lab Invest. 2002;82:345–51. doi: 10.1038/labinvest.3780428. [DOI] [PubMed] [Google Scholar]
- 27.Sarlomo-Rikala M, Tsujimura T, Lendahl U, Miettinen M. Patterns of nestin and other intermediate filament expression distinguish between gastrointestinal stromal tumors, leiomyomas and schwannomas. Apmis. 2002;110:499–507. doi: 10.1034/j.1600-0463.2002.100608.x. [DOI] [PubMed] [Google Scholar]
- 28.Florenes VA, Holm R, Myklebost O, Lendahl U, Fodstad O. Expression of the neuroectodermal intermediate filament nestin in human melanomas. Cancer Res. 1994;54:354–6. [PubMed] [Google Scholar]
- 29.Li H, Cherukuri P, Li N, Cowling V, Spinella M, Cole M, Godwin AK, Wells W, DiRenzo J. Nestin is expressed in the basal/myoepithelial layer of the mammary gland and is a selective marker of basal epithelial breast tumors. Cancer Res. 2007;67:501–10. doi: 10.1158/0008-5472.CAN-05-4571. [DOI] [PubMed] [Google Scholar]
- 30.Kleeberger W, Bova GS, Nielsen ME, Herawi M, Chuang AY, Epstein JI, Berman DM. Roles for the stem cell associated intermediate filament Nestin in prostate cancer migration and metastasis. Cancer Res. 2007;67:9199–206. doi: 10.1158/0008-5472.CAN-07-0806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohike N, Sato M, Hisayuki T, Imataka H, Sato S, Wada Y, Saito K, Takahashi M, Tajiri T, Kunimura T, Morohoshi T. Immunohistochemical analysis of nestin and c-kit and their significance in pancreatic tumors. Pathol Int. 2007;57:589–93. doi: 10.1111/j.1440-1827.2007.02143.x. [DOI] [PubMed] [Google Scholar]
- 32.Yang X, Wu Q, Yu X, Xu C, Ma B, Zhang X, Li S, Lahn B, Xiang AP. Nestin expression in different tumors and its relevance to malignant grade. J Clin Pathol. 2007 doi: 10.1136/jcp.2007.047605. [DOI] [PubMed] [Google Scholar]
- 33.Ishiwata T, Friess H, Buchler MW, Lopez ME, Korc M. Characterization of keratinocyte growth factor and receptor expression in human pancreatic cancer. Am J Pathol. 1998;153:213–22. doi: 10.1016/S0002-9440(10)65562-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ishiwata T. Immunohistochemical and in situ Hybridization Analysis of Lumican in Colorectal Carcinoma. In: Hayat MA, editor. IMMUNOHISTOCHEMISTRY AND IN SITU HYBRIDIZATION OF HUMAN CARCINOMAS. Burlington, USA: Elsevier Academic Press; 2005. pp. 237–243. [Google Scholar]
- 35.Shimada S, Tsuzuki T, Kuroda M, Nagasaka T, Hara K, Takahashi E, Hayakawa S, Ono K, Maeda N, Mori N, Illei PB. Nestin expression as a new marker in malignant peripheral nerve sheath tumors. Pathol Int. 2007;57:60–7. doi: 10.1111/j.1440-1827.2006.02059.x. [DOI] [PubMed] [Google Scholar]
- 36.Pino MS, Shrader M, Baker CH, Cognetti F, Xiong HQ, Abbruzzese JL, McConkey DJ. Transforming growth factor alpha expression drives constitutive epidermal growth factor receptor pathway activation and sensitivity to gefitinib (Iressa) in human pancreatic cancer cell lines. Cancer Res. 2006;66:3802–12. doi: 10.1158/0008-5472.CAN-05-3753. [DOI] [PubMed] [Google Scholar]
- 37.Maruyama H, Kleeff J, Wildi S, Friess H, Buchler MW, Israel MA, Korc M. Id-1 and Id-2 are overexpressed in pancreatic cancer and in dysplastic lesions in chronic pancreatitis. Am J Pathol. 1999;155:815–22. doi: 10.1016/S0002-9440(10)65180-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wildi S, Kleeff J, Maruyama H, Maurer CA, Friess H, Buchler MW, Lander AD, Korc M. Characterization of cytokeratin 20 expression in pancreatic and colorectal cancer. Clin Cancer Res. 1999;5:2840–7. [PubMed] [Google Scholar]
- 39.Itakura J, Ishiwata T, Friess H, Fujii H, Matsumoto Y, Buchler MW, Korc M. Enhanced expression of vascular endothelial growth factor in human pancreatic cancer correlates with local disease progression. Clin Cancer Res. 1997;3:1309–16. [PubMed] [Google Scholar]