Abstract
Agmatine, an endogenous amine derived from decarboxylation of L-arginine catalyzed by arginine decarboxylase, has been proposed as a neurotransmitter or neuromodulator in the brain. In the present study we examined whether agmatine has neuroprotective effects against repeated immobilization-induced morphological changes in brain tissues and possible effects of immobilization stress on endogenous agmatine levels and arginine decarboxylase expression in rat brains. Sprague-Dawley rats were subjected to two hour immobilization stress daily for seven days. This paradigm significantly increased plasma corticosterone levels, and the glutamate efflux in the hippocampus as measured by in vivo microdialysis. Immunohistochemical staining with β-tubulin III showed that repeated immobilization caused marked morphological alterations in the hippocampus and medial prefrontal cortex that were prevented by simultaneous treatment with agmatine (50 mg/kg/day, i.p.). Likewise, endogenous agmatine levels measured by high performance liquid chromatography in the prefrontal cortex, hippocampus, striatum and hypothalamus were significantly increased by immobilization, as compared to controls. The increased endogenous agmatine levels, ranging from 92% to 265% of controls, were accompanied by a significant increase of arginine decarboxylase protein levels in the same regions. These results demonstrate that administration of exogenous agmatine protects the hippocampus and medial prefrontal cortex against neuronal insults caused by repeated immobilization. The parallel increase in endogenous brain agmatine and arginine decarboxylase protein levels triggered by repeated immobilization indicates that the endogenous agmatine system may play an important role in adaptation to stress as a potential neuronal self-protection mechanism.
Keywords: agmatine, arginine decarboxylase, β-tubulin III, hippocampus, immobilization
1. Introduction
Stress is a common experience of daily life. Physical and psychological stressors elicit integrated neuroendocrine and autonomic responses, including an enhanced activity of the hypothalamic-pituitary-adrenal axis with elevated circulating glucocorticoid concentrations. These responses serve to mobilize and redistribute bodily resources to facilitate and maintain physiological homeostasis. While this interaction of hormonal and neuronal systems is a fundamental requirement for survival, there is considerable evidence that high levels of glucocorticoids in the brain resulting from prolonged stress may produce deleterious effects, including damage to neurons (McIntosh and Sapolsky 1996). In fact, animal studies have shown that prolonged exposure to high levels of corticosterone has diverse effects on the central nervous system, including changes in cellular activity, neurochemistry, and neuronal morphology (Sapolsky et al. 1985; Sapolsky 1990; Reagan and McEwen 1997).
It has been known for some time that excitatory amino acids, especially glutamate, are also strongly implicated in stress-induced structural and functional changes in the rat brain. An increased extracellular glutamate level in the rat brain regions caused by both acute restraint and swimming stress was reported (Lowy et al. 1993; Moghaddam 1993). Also N-methyl-D-aspartate (NMDA) receptor antagonists can block stress-induced structural changes in the hippocampus (Magarinos et al. 1999). These findings not only suggest a possible role for glutamate in the mechanisms underlying cellular and molecular alterations caused by stress, but also provide an avenue to block or attenuate chronic stress-induced effects by normalizing glutamatergic function during stressful stimuli. Such an approach might have distinct clinical advantages.
Agmatine is an endogenous cationic amine derived from enzymatic decarboxylation of L-arginine by arginine decarboxylase (ADC, EC4.1.1.19) (Tabor and Tabor 1984). In vivo, agmatine is enriched in the hippocampus and other brain regions (Regunathan et al. 1995; Feng et al. 1997; Otake et al. 1998). An important enzyme for biosynthesis of agmatine, ADC is widely expressed in many brain regions of rats and human and this regional distribution may adumbrate some of agmatine's functional effects. In the past decade, many studies have demonstrated that agmatine has neuroprotective effects in vitro and in vivo. Our previous work has also demonstrated neuroprotective effects of agmatine against cell damage caused by glucocorticoids and glutamate in primary neuronal cultures of the hippocampus (Zhu et al. 2006). That study indicated that agmatine may play a role in the homeostasis during stress, particularly with regard to stress effects that are mediated by glucocorticoids and glutamate.
We have recently reported that chronic treatment of rats with glucocorticoids resulted in morphological changes in the hippocampus and medial prefrontal cortex (mPFC). These morphological changes can be prevented by administration of agmatine (Zhu et al. 2007). To test the hypothesis that the previously observed pathological impacts of chronic exposure to glucocorticoids are similar to the neuronal insults caused from chronic stress, we carried out the present study to determine whether repeated immobilization causes morphological alterations in rat brains and whether agmatine can protect brain tissues from immobilization-induced cytoarchitectural alterations. As glutamate release has been indicated to contribute to the deleterious effects of chronic stress, we also measured extracellular glutamate levels in this animal model. Furthermore, we measured endogenous agmatine and ADC protein levels in brain regions of rats subjected to immobilization stress in order to determine whether repeated immobilization affects endogenous agmatine levels in related brain regions. Our results reveal the neuroprotective effects of agmatine and potential roles of agmatine systems in the adaptation response to chronic stress.
2. Materials and Methods
2.1. Animals
Male Sprague-Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) weighing 200-250 g at the time of initiation of immobilization stress were housed on a 12 hours light/dark cycle with food and water provided ad libitum. All animal procedures were approved by the Animal Care and Use Committee of East Tennessee State University and University of Mississippi Medical Center and complied with the NIH Guide for the Care and Use of Laboratory Animals. After an acclimation period of 5 days, rats were randomly assigned to experimental groups.
2.2. Stress paradigm
The animals were randomly assigned to chronic immobilization or control groups. Immobilization stress was accomplished by taping the four limbs of rats to a wooden board with two metal loops around the neck for two hours a day (10.00 am to 12.00 pm) for seven consecutive days. Control animals were brought to the same room every day without immobilization (sham). During the actual or sham immobilization sessions there was no access to either food or water.
Four groups of rats were used in the immunostaining study: control, two groups of immobilization stress alone, and immobilization stress plus agmatine treatment. Rats in the control and stress alone groups were daily injected with saline intraperitoneally. Rats in the stress plus agmatine group were administered with agmatine daily (50 mg/kg, i.p.), immediately before beginning of the immobilization paradigm. The choice of agmatine dose is based on a previous study where administration of exogenous agmatine (50 mg/kg, i.p.) to rats increased agmatine levels in the hippocampus and frontal cortex ∼2-4-fold at 15-30 min after injection (Feng et al. 2005), demonstrating that agmatine accesses the brain and this dose of agmatine is enough high to raise the agmatine levels in the brain. As our previous study showed that administration of agmatine at the dose of 50 mg/kg did not produce any morphological alteration in the hippocampus and mPFC (Zhu et al. 2008), we did not include this group in the present study. Immediately after the last immobilization session (on the 7th day), rats in the control, one group of immobilization (Immo-1) and immobilization plus agmatine were transcardically perfused under pentobarbital anesthesia using 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. Rats in another group of immobilization (Immo-2) were perfused by the same way but two hours after the last immobilization session. Brains were further stored in 10% sucrose followed by 30% sucrose, and then sectioned at 30 μm using a sliding microtome for subsequent immunostaining.
There were three groups of rats in the studies measuring endogenous agmatine and ADC protein expression: control and two groups of immobilization (similarly, Immo-1 and Immmo-2). As the goal of this experiment was to determine the effects of immobilization on endogenous agmatine and ADC levels, there was no agmatine treatment group in this experiment. After the last immobilization session (on the 7th day), rats in the group of control and Immo-1 were immediately sacrificed by decapitation. Rats in the group Immo-2 were sacrificed two hours after the last immobilization session. Rat brain regions were rapidly dissected on ice and immediately processed for high performance liquid chromatography (HPLC) or ADC assays.
2.3. Rat blood sampling and plasma corticosterone determination
On the 7th day after the last session of immobilization stress, rats were sacrificed. The period of time between removing rats from the wooden board to decapitation was strictly held to 15 seconds or less. Trunk blood from rats was quickly collected into chilled glass tubes, which have been rinsed with a solution of 1.5% EDTA in saline and dried. Animals in the control group were sacrificed by the same procedure. Blood samples were immediately centrifuged at 2500 rpm. Plasma obtained was temporarily stored at -20°C and plasma corticosterone was later measured by a radioimmunoassay using a commercial kit [ImmuChem radioimmunoassay kit, MP Biomedicals, LLC in Orangeburg, NY (formerly ICN Pharmaceuticals, Costa Mesa, CA)]. The assay kit was used according to manufacturers instructions with the following modification: all reagents are used at half strength and the lowest standard concentration in the kit (25 ng) was serially diluted to yield standards at 12.5, 6.25 and 3.125 ng, respectively. The resulting assay has a sensitivity of 3.125 ng and an IC50 of 65 ng. All samples were run in a single assay with an intra-assay variance of less than 8%.
2.4. Surgery and in vivo microdialysis
This study included two groups of rats: control and immobilization. The surgical procedures and microdialysis sampling were performed using modifications of techniques detailed as previous studies (Feng et al. 2005). Briefly, rats were anesthetized with halothane inhalation, and a CMA/11 microdialysis guide cannula (CMA Microdialysis, N. Chelmsford, MA) was implanted with the tip directed toward the left ventral hippocampus. The coordinates for implantation were (in mm relative to bregma): lateral 5.0 and posterior 5.2 (Paxinos and Watson, 2005). The cannula tip was lowered 4.5 mm below the surface of the dura. Five stainless steel screws were secured to the skull. An aluminum protective cap was placed around each guide cannula and dental acrylic (Lang Dental, Wheeling, IL) was applied to anchor the assembly to the skull surface. Operated rats were allowed 7 days to recover. All animals used in the study displayed no signs of distress as a result of surgery (i.e., they moved and groomed normally, they ate food and drank water, and there was no sign of bleeding or infection around the surgery area). Microdialysis was initiated by the insertion of microdialysis probes (CMA/11, 2 mm tip) into the guide cannula when the rat was placed in an individual CMA/120 system under freely moving conditions at 8.00 am on the 7th day of stress paradigm. The artificial cerebrospinal fluid (aCSF, composition:140 mM NaCl, 2.7 mM KCl, 1.2 mM CaCl2, 1 mM MgCl2, 0.3 mM NaH2PO4, 2.7 mM Na2HPO4, pH 7.4) was continuously perfused through the probe at 1 μl/min by a CMA pump for an equilibration period of two hours. A series of four sequential 15 min samples for each rat was taken prior to immobilization to demonstrate a steady baseline. These four samples were averaged and all subsequent values were expressed as a percentage of this pre-immobilization value (% of baseline). Then the rat was subjected to immobilization while dialysis was continued. Dialysate samples were collected and subsequently analyzed by HPLC as described previously (Feng et al. 2005). After completion of microdialysis, rats under anesthesia with pentobarbital were perfused intracardially using 4% paraformaldehyde in 0.1 M phosphate buffer, pH 7.4. The brain was examined for probe placement. The rats in which the probe was not in the designated location were not included in statistical data. The in vitro recovery of glutamate was determined by immersion of probes in aCSF containing 10 μM of glutamate and ranged from 20 to 25% for glutamate.
2.5. Immunostaining with β-tubulin III
Immunocytochemical staining was performed using a monoclonal antibody to β-tubulin III raised in mice (Chemicon, Temecula, CA). Brain sections were washed three times in phosphate-buffered saline (PBS) and pre-incubated in 5% bovine serum in phosphate-buffered saline supplemented with 0.2% Triton-X 100 for one hour at room temperature (25°C), followed by incubation with the primary antibody (1:500 dilution, in PBS containing 0.2% Triton-X 100) overnight at 4°C. The following day, binding of β-tubulin III antibody was detected with a biotinylated secondary antibody (goat anti-mouse, 1:200) using ABC kit (Vector Laboratories, Burlingame, CA) according to the instructions of the manufacturer. β-Tubulin III immunoreactivity was visualized by incubating sections in 0.05% 3, 3′-diaminobenzidine tetrahydeochloride, 0.07% NH4NiSO4 and 0.003% H2O2. Staining for β-tubulin III was then analyzed microscopically using MagnaFire SP imaging software (Optromics, Goleta, CA). Some slides were processed to determine the specificity of this immunostaining using the same procedures as described above except that the primary antibody was not applied. No positive immunostaining was found in these slides (data not shown). In order to reduce inter-animal staining variability, each immunostaining experiment was performed on all tissue sections at the same time by an investigator blinded to the animal group.
2.6. Immunohistochemical analysis
β-Tubulin III immunoreactivity for dendrites was evaluated by the density analysis using ImageJ software (version 1.37) (Rasband, W.S., National Institutes of Health, Bethesda, MD, http://rsb.info.nih.gov/ij/, 1997-2006). Care was taken to ensure that the identical plane of sectioning from animals of different groups was used to ensure uniformity and exclude the possibility that the present results were an artifact of the sectioning plane. First, images were acquired in an Olympus BX41 microscope (Tokyo, Japan) equipped with an Olympus U-TVO digital camera connected to a computer with MagnaFire image software (Goleta, CA). Three digital microscopic images under higher power (100× oil immersion objective lenses) were randomly captured at the CA1, CA2, and CA3 of the same hemisphere of hippocampus and the dorsal anterior cingulated cortex (ACd), prelimbic cortex (PL) and infralimbic cortex (IL) of the mPFC. The image size of the view field was ∼75 μm2. These grey-scale images were thresholded in ImageJ with a fixed level over the background. The threshold values for immunoreactive dendrites were obtained by manually sampling the signal intensity in each image, which was visually compared with the original grey-scale images to ensure that the tool effectively resolved all labeled fibers. Thereafter, the intensity of β-tubulin III immunoreactivity (dendrites) in the area was semi-quantitatively obtained using the measurement tool of ImageJ and the target (above threshold) area was expressed as a percentage of the sampled area. Two sections (around Bregma -3.24 mm for the hippocampus, and near Bregma +3.24 mm for the mPFC) from each animal were examined. Therefore, for each animal, about 6 measurements were obtained from each of the CA1, CA2, CA3 and mPFC subfield regions. The average of the measurements (percent areas) obtained in 6 rats from each group was a reflection of staining intensity for the hippocampus or the sub-regions of the mPFC.
2.7. Measurement of agmatine levels in brain tissues
The amount of agmatine in brain tissues in the control and stressed rats was measured by HPLC as described previously (Raasch et al. 1995; Feng et al. 1997). Brain tissues were homogenized after dissection and re-suspended in the phosphate buffer (pH 5.7) containing amino picollinate as an internal standard. The cell suspension was then homogenized in 10% trichloroacetic acid and centrifuged. The supernatant was used for HPLC analysis. Samples were derivatized with o-phthalaldehyde and loaded onto a reverse phase HPLC column (five μm) connected with a fluorescence detector. The recovery of agmatine was calculated from an added external standard and expressed as μg/g wet tissue weight.
2.8. Detection of ADC protein levels
ADC protein levels in brain regions were determined by Western blotting using a rabbit polyclonal antibody that recognized a unique 17 amino acid ADC peptide as described previously (Iyo et al. 2006). The IgG fraction of this antiserum was purified using a protein A column (Pierce, Rockford, IL) and the purified IgG was characterized for its specificity by pre-absorption with an antigen peptide (Iyo et al. 2006). Rat brain regions were dissected and homogenized in HEPES buffer (pH 7.4). After centrifugation at 1000 ×g, the supernatants were centrifuged at 30,000 ×g for 20 min. The resulting pellets were solubilized in the sample buffer containing sodium lauryl sulfate (SDS) for ADC immunoblotting analysis. Equal amounts of homogenized proteins were loaded on a 10% SDS-polyacrylamide gel for electrophoresis and transferred by electro-blotting to a polyvinylidene diflouride membrane. The ADC antibody was applied to the membrane at a dilution of 1:3000 to detect the ADC protein. Membranes were processed for immuno-blot analysis using the enhanced chemilumiscence (ECL) detection system (Amersham, Piscataway, NJ). Immunoreactive bands were visualized using the Kodak Image station (New Haven, CT). The measurement of Western blot protein band densities was performed on tissues of five rats from each group by analyzing using imaging software (Molecular Dynamics IQ Solutions, Molecular Dynamics, Inc., Sunnyvale, CA). A linear standard curve was created from optical densities (ODs) of bands with a dilution series of total proteins prepared from saline-treated rats on each blot. The OD values of ADC sample bands were compared with those of the standard curve to ensure that detection was in the linear range of measurement. The OD values of immunoreactive ADC were further normalized by dividing by the OD values for immunoreactive β-actin determined on the same blot.
2.9. Statistics
Data are presented as means ± SEM. For data from the measurements of plasma corticosterone and hippocampal glutamate, a student t-test was used. Data from ImageJ analysis, the measurement of endogenous agmatine and ADC protein levels in brain regions, were analyzed by one-way analysis of variance (ANOVA, SigmaStat, SYSTAT Software, Inc, Richmond, CA). In the presence of significant F values, individual comparisons between the means were made using the Student-Newman-Keuls test.
3. Results
3.1. Repeated immobilization increased plasma corticosterone and extracellular glutamate levels in the hippocampus
This experiment was carried out to define the efficacy of repeated immobilization in inducing stress-like modification in rats. First, plasma corticosterone levels were measured in samples collected immediately after two hour immobilization period at the 7th stress day. Plasma corticosterone levels were significantly elevated in rats subjected to repeated immobilization (50.19±9.16 μg/dl) compared to control rats (10.47±4.16 μg/dl; p<0.01). Similarly, as our previous study showed that administration of agmatine (50 mg/kg, i.p.) did not affect plasma corticosterone levels in control and stress rats (Zhu et al. 2008), we did not repeat these measurement in the present study. Second, in vivo microdialysis was performed during the two-hour immobilization period at the 7th stress day to measure extracellular glutamate levels in the hippocampus. As shown in Figure 1, immobilization stress resulted in a significant increase in extracellular glutamate levels in the hippocampus at all time points except for the last one after the initiation of immobilization, as compared to those of control rats. Glutamate levels peaked with a four-fold increase at 15 minutes (p<0.01) after immobilization and then gradually dropped, although remaining about 50% to 90% higher (all p<0.05) than those in the control group.
Figure 1.
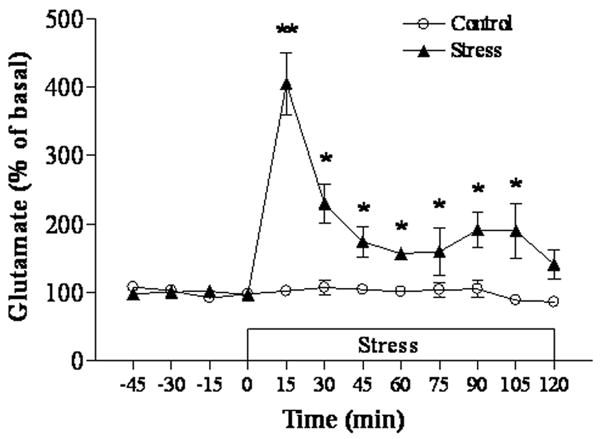
Effects of immobilization on extracellular glutamate in the hippocampus (n=5 for each group). The basal level of glutamate is 18.94 ± 1.21 μM. * p<0.05, ** p<0.01 compared to the control.
3.2. Administration of agmatine prevented immobilization-induced morphological alteration in the hippocampus and mPFC
We next used immunocytochemical staining of β-tubulin III to examine the effects of repeated immobilization on the morphology of neurons in the hippocampus and mPFC of rat brains. In order to distinguish whether chronic or acute stress affects the brain morphology, immobilized rats were perfused immediately (Immo-1) or two hours (Immo-2) after the last stress session. As the results from two groups were very similar, we described their morphological alteration together and also did not respectively show the semi-quantitative results. As shown in Figure 2, dendrites of the hippocampus in control animals showed a homogeneous distribution of β-tubulin III with intensive immunostaining. In contrast, there was a marked reduction of immunoreactive-staining in dendrites in CA1 and CA2 regions in rats subjected to repeated immobilization (both Immo-1 and Immo-2). These less intensively immunostained dendrites appeared twisted or broken and displayed a disoriented morphology. Similarly, immunostaining of mossy fiber terminals in the stratum lucidum of CA3 showed a marked reduction with a shorter dendritic length and lower density. However, in stressed rats pre-treated with agmatine (50 mg/kg, i.p.), the morphology of CA1, CA2 and CA3 was almost the same as those of the control (Figure 2), indicating that treatment with agmatine prevented the cytoarchitectural alterations caused by repeated immobilization.
Figure 2.
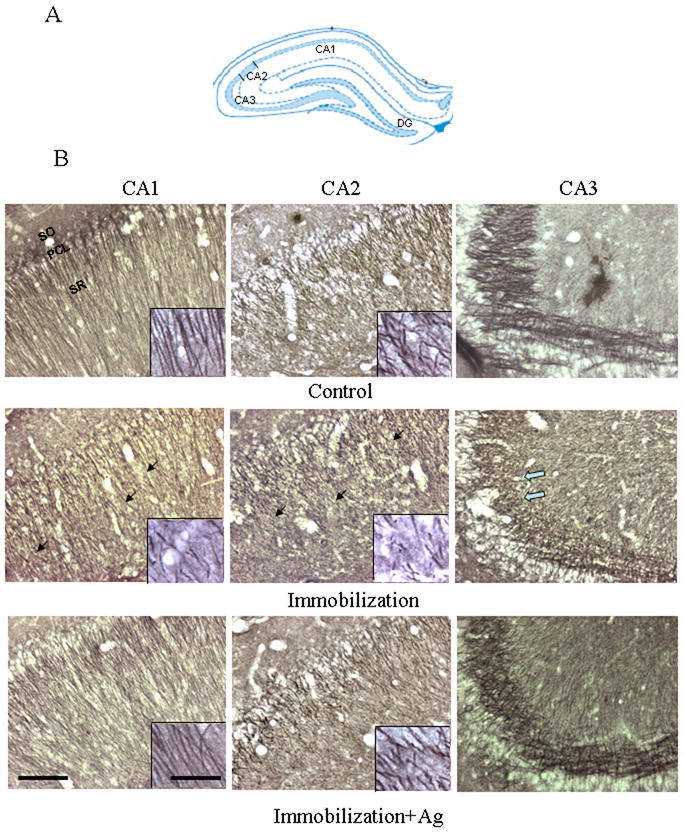
A. Schematic representation of the hippocampal subfields in the coronal plane (approximately Bregma -3.24 mm) (Paxinos and Watson 2005). B. Photomicrographs showing the CA1, CA2 and CA3 of the rat hippocampus immunostained with β-tubulin III after the injection of saline (Control), repeated immobilization plus saline (Immobilization) and repeated immobilization plus agmatine (50 mg/kg/day, i.p.) (Immobilization+Ag) for seven days (n=7 for each group). PCL, pyramidal cell layer; SO, stratum oriens; SR, stratum radiatum. Scale bars = 100 μm for the pictures except for inserts; 20 μm for inserts. Solid arrows point to areas where showed less intensity of immunostaining in the CA1 and CA2 of stress animals, compared to those of the control and immobilization plus agmatine. The open arrow points to an area in the CA3 with reduced mossy fibers in stress animals.
In control animals, immunostained dendrites of the anterior cingulate cortex (ACd) and both the pre-limbic (PL) and infra-limbic (IL) sub-divisions of the mPFC appeared as long, branching processes (Figure 3). However, dysmorphic changes resembling those of the hippocampus could be seen in these cortical areas of rats subjected to repeated immobilization (Immo-1 and Immo-2). Even with less dense immunostaining, dendritic fibers of the mPFC in the immobilization group became shorter, and more irregular than those of the control group. Pre-treatment with agmatine (50 mg/kg, i.p.) prevented the loss of β-tubulin III immunostaining in these stress animals, which appeared to have nearly as normal dendritic morphology as was seen in the control group. No obvious structural changes were observed in the striatum and hypothalamus after stress (data not shown).
Figure 3.
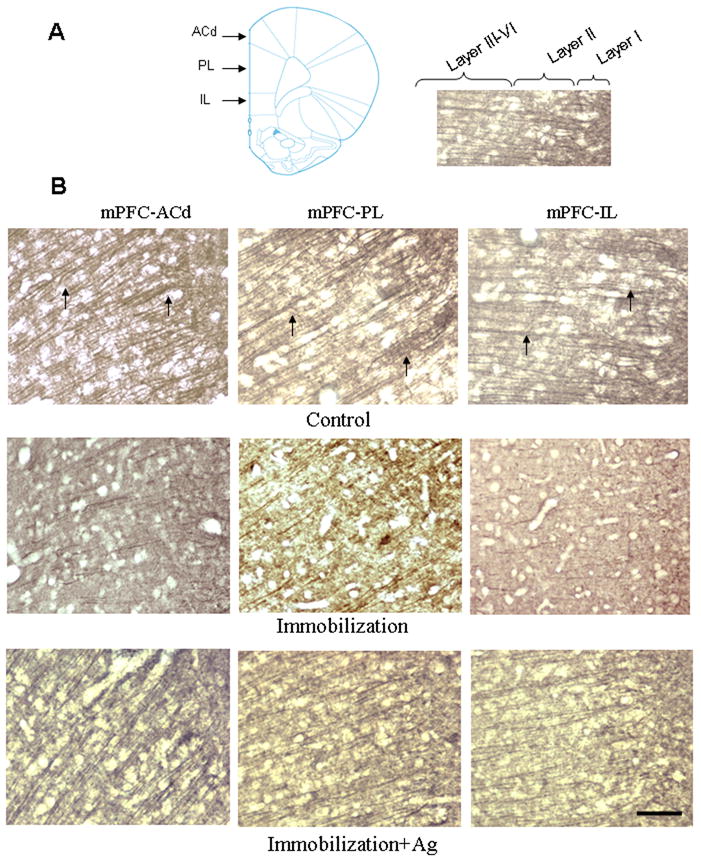
A. Left: Schematic representation of the division of the mPFC in the coronal plane (approximately Bregma +3.24 mm; Paxinos and Watson, 2005). Right: the same image of mPFC-IL showing boundaries between layers. B. Photomicrographs showing the dorsal anterior cingulate cortex (ACd), prelimbic cortex (PL) and infralimbic cortex (IL) in the rat mPFC immunostained with β-tubulin III antibody after injection of saline (Control), repeated immobilization plus saline (Immobilization) and repeated immobilization stress plus agmatine (50 mg/kg/day, i.p.) (Immobilization+Ag) for seven days (n=7 for each group). Arrows indicate apical dendrites immunostained for β-tubulin III. Scale bar = 100 μm for all images.
We further semi-quantitatively examined β-tubulin III immunoreactive intensities for dendrites in the sections of the hippocampus and mPFC from experimental animals. An one-way ANOVA analysis revealed significant differences in β-tubulin III immunoreactivities in the CA1 (F2,13= 14.81, p<0.01), CA2 (F2,11= 43.34, p<0.001) and CA3 (F2,8= 14.55, p<0.01) in the hippocampus, as well as in the regions of ACd (F2,11= 14.45, p<0.01), PL (F2,11= 47.77, p<0.001) and IL fields (F2,11= 48.22, p<0.001) of the mPFC from rats of the control, immobilization alone or in combination with agmatine treatment. Post hoc analysis revealed that repeated immobilization significantly reduced β-tubulin III immunoreactive intensity in CA1, CA2 and CA3 by 22%, 35% and 29% (all p<0.01), respectively, as compared to the control. Treatment with agmatine prevented this reduction in these regions, as compared to the repeated immobilization alone (Table 1). Similarly, repeated immobilization significantly reduced β-tubulin III immunoreactivity in the regions of ACd by 31% (p<0.01), PL by 25% (p<0.01) and IL by 31% (p<0.01) of the mPFC. Likewise, treatment with agmatine abolished the effect of repeated immobilizaiton on β-tubulin III immonoreactive intensity in the ACd, PL and IL areas of the mPFC (all p<0.01), as compared to the group of repeated immobilization alone.
Table 1.
Semi-quantitative analysis of β-tubulin III immunoreactive intensities (% Area) in the hippocampus and mPFC of rats.
| Hippocampus | mPFC | |||||
|---|---|---|---|---|---|---|
| CA1 | CA2 | CA3 | ACd | PL | IL | |
| Control | 47.22±1.94 | 47.09±1.50 | 51.35±0.66 | 34.92±2.13 | 28.61±0.88 | 31.41±1.07 |
| Immobilization | 36.84±1.16*† | 30.44±1.46*† | 36.23±0.19*† | 24.19±1.04*† | 21.42±0.20*† | 21.75±0.42*† |
| Immobilization + Agmatine | 46.65±1.04 | 45.48±1.21 | 50.01±0.98 | 33.17±1.12 | 27.64±0.30 | 29.58±0.57 |
Results are expressed as mean ± S.E.M. (n=6 for each group),
p<0.01, compared to the control;
p<0.01, compared to the group of repeated immobilization plus agmatine. The individual comparisons were done by the One-way ANOVA plus Student-Newman-Keuls test.
3.3. Repeated immobilization increased endogenous agmatine levels in brain regions
Further, HPLC measurement was performed to detect whether repeated immobilization affects endogenous agmatine levels in the ex-vivo hippocampus and mPFC. As shown in Figure 4, seven-day repeated immobilization significantly increased endogenous agmatine levels in all four brain regions (prefrontal cortex: F2,20 = 6.24, p<0.05; hippocampus: F2,20 = 7.87, p<0.05; striatum: F2,20 = 11.25, p<0.01; hypothalamus: F2,20 = 14.68, p<0.01). Post hoc analysis revealed that chronic immobilization increased agmatine levels in the prefrontal cortex (P<0.05) and hippocampus (P<0.05) by 95 % and 92%, respectively. Furthermore, endogenous agmatine levels in the striatum and hypothalamus were increased after repeated immobilization by 215% and 265% (both p<0.01), respectively, compared to those of the control animals. There was no difference in endogenous agmatine levels between the groups of Immo-1 and Immo-2.
Figure 4.
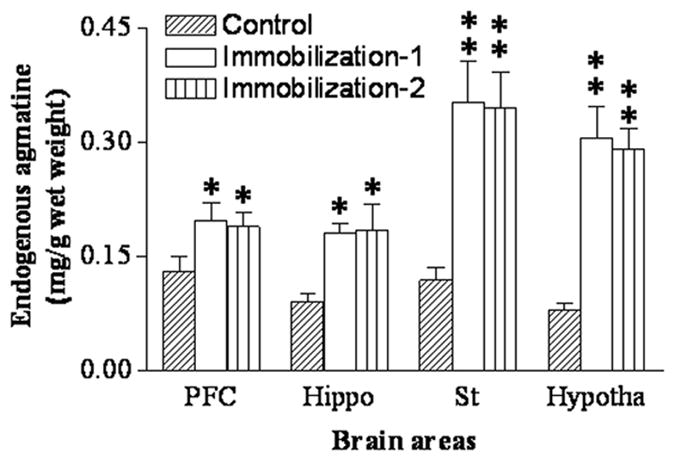
Endogenous agmatine concentrations measured by HPLC in the prefrontal cortex (PFC), striatum (St), hypothalamus (Hypotha) and hippocampus (Hippo) in control and repeated immobilization rats (Immo-2 and Immo-2) (for the group of control and Immo-1: n=8; for the group of Immo-2: n=5). Results are expressed as the group mean ± SEM. * p<0.05, ** p<0.01, compared to the control.
3.4. The effects of immobilization on ADC levels in rat brain areas
ADC is an important enzyme for synthesis of endogenous agmatine. In the present study, ADC protein levels were measured to investigate effects of immobilization on this enzyme in various brain areas of rats. Semi-quantitative analysis of Western blots by one-way ANOVA revealed that repeated immobilization significantly increased ADC protein levels in all four brain regions: the prefrontal cortex (F2,17=26.23, p<0.01; Figure 5A), hippocampus (F2,17=10.54, p<0.05; Figure 5B), striatum (F2,17 =16.92, p<0.01; Figure 5C), and hypothalamus (F2,17 =6.97, p<0.05; Figure 5D). Post hoc analysis revealed both ADC levels in the groups of Immo-1 and Immo-2 were significantly higher than that of the control (p<0.05), indicating that the alteration in ADC levels of these brain regions was caused by chronic stress.
Figure 5.
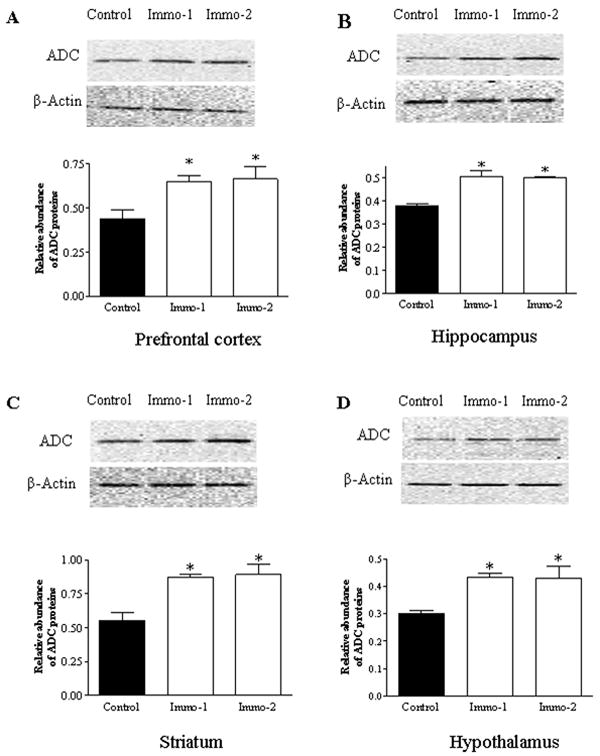
Upper panel: Photograph of immunoblotted ADC in the PFC (A), hippocampus (B), striatum (C) and hypothalamus (D) in control and repeated immobilization rats (for the group of control and Immo-1: n=7; for the group of Immo-2: n=4). Middle panel: The same membrane probed again with anti-β-actin antibody (after stripping) as an internal control. Lower panel: Semi-quantitative analysis by densitometry of bands after Western blotting. Results are the mean ± SEM (n=5). * p<0.05, compared to the control.
4. Discussion
Our previous study demonstrated that agmatine has neuroprotective effects against glucocorticoid-induced neuronal damage in vitro (Zhu et al. 2006) and in vivo (Zhu et al. 2007), as identified by immunohistochemical staining for β-tubulin III. The in vivo study also revealed that there is a possible regulatory effect of glucocorticoids on agmatine synthesis, because treatment with glucocorticoids resulted in a proportional alteration between endogenous agmatine and ADC protein levels (Zhu et al. 2007). This regulation may represent a portion of the adaptation responses to stress and underlie a major physiological role of agmatine in the brain. In this paper, we sought to test this hypothesis using an animal stress model of repeated immobilization. The results reveal that with elevated plasma corticosterone levels and a significant high glutamate efflux in the hippocampus, repeated immobilization caused marked architectural alterations in the hippocampus and mPFC, which can be prevented by simultaneous treatment with exogenous agmatine. Biochemical measurements demonstrated that repeated immobilization also significantly increased endogenous agmatine levels and ADC protein expression in the frontal cortex and hippocampus, as well as in the striatum and hypothalamus. It can be inferred from these results that the endogenous agmatine system may play an important role in modulating biological responses to chronic stress. Our finding is consistent to a presumption that agmatine acts as an endogenous modulation in the mood (Dias Elpo Zomkowski et al. 2004), as administration of agmatine produces antidepressant-like effect in the stress models of mice and rats (Zomkowski et al. 2002; Li et al. 2003), and anxiolytic effect in rats (Lavinsky et al. 2003).
Many studies have revealed that chronic stress affects synaptic plasticity and dendritic morphology in the hippocampus (Watanabe et al. 1992; Magarinos and McEwen 1995; Sunanda et al. 1995; Galea et al. 1997; Sousa et al. 2000) and mPFC (Cook and Wellman 2004; Radley et al. 2004; Radley et al. 2006). In accordance with these previous observations, the present study demonstrated that repeated immobilization markedly reduced immunoreactive dendritic densities in the hippocampus and mPFC, as identified by immunostaining with β-tubulin III. However, whether the structural alterations in the hippocampus and mPFC identified by β-tubulin III immunostaining are reversible is needed to be further studied. Nevertheless, the present immunostaining results, coupled with those performed in rats treated with glucocorticoids (Zhu et al. 2007) or subjected to repeated restraint stress (Zhu et al. 2008), suggest that the changes in β-tubulin III immunostaining is a putative proxy of alterations in neuronal morphology induced by chronic stress.
Despite extensive efforts, the precise mechanisms underlying chronic stress-induced neuronal damage are still a matter of debate. There is considerable evidence that stress-induced increases of corticosterone in brain tissues are factors that mediate deleterious effects of chronic stress (Moghaddam et al. 1994; Magarinos and McEwen 1995). The neurotransmitter, glutamate, is also likely involved in the pathogenesis of stress-induced brain damage (Gilad et al. 1990; Lowy et al. 1993; Moghaddam 1993). A modest increase in glucocorticoid concentrations that occurs between the trough and peak part of the normal circadian cycle doubles extracellular glutamate concentrations, while a high increase in glucocorticoid levels induced by stress produces a four-fold increase in glutamate (Stein-Behrens et al. 1994). It has been postulated that glucocorticoid-induced elevations in extracellular glutamate may account for the deleterious effects of stress on brain neurons (Moghaddam et al. 1994). The present study showed a significantly high level of plasma corticosterone, and a high glutamate efflux in the hippocampus in rats subjected to repeated immobilization, as measured by in vivo microdialysis (Figure 1). These results are consistent with previous investigations regarding elevated plasma corticosterone (Watanabe et al. 1992; Madrigal et al. 2003; Retana-Marquez et al. 2003; Marquez et al. 2004) and extracellular glutamate levels in brains of stressed animals (Lowy et al. 1993; Moghaddam 1993; Steciuk et al. 2000), although the latter measurements for glutamate were performed in rats subjected to acute stress. The findings of the present study, together with earlier evidence from other studies, indicate a cause-effect relationship between elevated glutamate levels and repeated immobilization-induced architectural alteration in the hippocampus and mPFC.
Agmatine has been reported to exert neuroprotective action by reducing the size of ischemic infarctions or preventing the loss of cerebella neurons after focal or global ischemia in vivo (Gilad et al. 1996; Kim et al. 2004). Our previous works also demonstrated that agmatine protects neurons against cell damage caused by glutamate and glucocorticoids in vitro (Zhu et al. 2003; Wang et al. 2006) and in vivo (Zhu et al. 2007). Interestingly, the repeatedly immobilized rats simultaneously treated with exogenous agmatine showed an almost normal morphology in the hippocampus and mPFC (Figures 2 and 3), indicating that exogenous agmatine prevents repeated immobilization-induced cytoarchitectural alterations. Several potential mechanisms may contribute to agmatine's neuroprotective effects. First, agmatine can block voltage-gated calcium channels with high potency in cultured rat hippocampal neurons (Weng et al. 2003; Zheng et al. 2004). Second, repeated immobilization induces the expression of several cytokines in brains (Minami et al. 1991; Shintani et al. 1995), including inducible nitric oxide synthases (NOSs) (Olivenza et al. 2000). These NOSs may contribute to the cell damage associated with chronic stress. Agmatine, by inhibiting all isoforms of NOS (Auguet et al. 1995; Galea et al. 1996), should reduce this cell damage. Finally, agmatine carries a guanidine group that enables it to block NMDA receptor channels (Armanini et al. 1990; Yang and Reis 1999). As repeated immobilization produced an increased glutamate efflux in the hippocampus of rats (Figure 1), this ability of exogenously administered agmatine to block NMDA receptor may account for its neuroprotective ability.
The present study showed that agmatine levels in four measured brain regions were significantly increased after seven-day immobilization, which is in agreement with a previous report (Aricioglu et al. 2003). These increased endogenous agmatine levels were accompanied by a significant increase in protein levels of ADC, the enzyme responsible for the synthesis of agmatine in the brain (Tabor and Tabor 1984; Iyo et al. 2006). Therefore, one might postulate that as one element of self-protection mechanisms in the brain, agmatine synthesis is trigged by repeated immobilization through activation of ADC expression, which in turn increases endogenous agmatine levels as an initial protective response to stress. However, the immunostaining results seem to be paradoxical to this postulation, because repeated immobilization still resulted in cytoarchitectural alterations in the hippocampus and mPFC, despite increased endogenous agmatine levels. Nevertheless, administration of exogenous agmatine did effectively prevent these alterations (Figures 2 and 3). One possible explanation is that increased endogenous agmatine levels in the hippocampus and mPRC trigged by repeated immobilization are not high enough to against deleterious effects of repeated immobilization. This notion is supported by our previous study (Feng et al. 2005). In that study, injection of agmatine, at the dose of 50 mg/kg which was also used in the present study, produced tissue levels of agmatine around 0.48 ± 0.08 and 0.69 ± 0.12 μg/g wet weight in the rat hippocampus and frontal cortex, respectively. These agmatine levels are much higher (2.67 to 3.83 times) than the measured endogenous agmatine levels of the stressed rats in the present study (0.18 ± 0.03 and 0.198 ± 0.03 μg/g wet weight in the hippocampus and mPFC, respectively), although the latter levels remained significantly higher than those of controls (0.09 ± 0.01 and 0.11 ± 0.01 μg/g wet weight, respectively). By comparison, endogenous agmatine levels in the striatum and hypothalamus were greatly elevated after repeated immobilization (0.37 ± 0.09 and 0.33 ± 0.08 μg/g wet weight, respectively, Figure 4), relative to the increases in the hippocampus and mPFC. Coincidentally, there is no noticeable architectural alteration in the striatum and hypothalamus after repeated immobilization. Whether this is due to differences in agmatine levels in these regions after immobilization stress is not known. Further study is warranted.
It is noteworthy that the immobilization paradigm used in the present study, in which movements of rats were restricted by taping the four limbs to a wooden board (four-point immobilization), is different from that of restraint stress in which rats were put in plastic restrainers or restraint bags. Although the restraint paradigm was also called immobilization sometimes (Olivenza et al. 2000; Vyas et al. 2002; Nadeem et al. 2006), it was less stressful than that of four-point immobilization according to the previous report (Dal-Zotto et al. 2003) and our observation in the present study. This difference can also be verified by the morphological results. Seven-day restraint caused no morphological changes in the hippocampus and mPFC (Zhu et al., 2008), while seven-day of repeated immobilization resulted in marked architectural alterations in the same regions (Figures 2, 3 and Table 1). The reason for this discrepancy may at least partially lie in the nature of repeated immobilization. Repeated immobilization is a strong stressor with higher-intensity and characterized by a prolonged post-stress maintenance of glucocorticoid secretion and anorexia (Marquez et al. 2002). Another possible explanation is that repeated immobilization caused a significantly high glutamate efflux (Figure 1), though there is no such measurement in restraint stress study. Further investigation is needed to elucidate these differences.
In conclusion, the present study demonstrated that repeated immobilization caused cytoarchitectural alterations in the hippocampus and mPFC, possibly as a consequence of stress-induced hypercortisolemia and high glutamate efflux. While exogenous administration of agmatine prevents these neuronal insults, the activation of ADC expression and subsequent increases of endogenous agmatine levels following repeated immobilization may be an important homeostatic adaptation of the brain. The findings suggest that endogenous agmatine may play an important role in neuroprotective mechanisms.
Acknowledgments
This work is supported by NIH grant RR17701.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aricioglu F, Regunathan S, Piletz JE. Is agmatine an endogenous factor against stress? Ann N Y Acad Sci. 2003;1009:127–132. doi: 10.1196/annals.1304.012. [DOI] [PubMed] [Google Scholar]
- Armanini MP, Hutchins C, Stein BA, Sapolsky RM. Glucocorticoid endangerment of hippocampal neurons is NMDA-receptor dependent. Brain Res. 1990;532:7–12. doi: 10.1016/0006-8993(90)91734-x. [DOI] [PubMed] [Google Scholar]
- Auguet M, Viossat I, Marin JG, Chabrier PE. Selective inhibition of inducible nitric oxide synthase by agmatine. Jpn J Pharmacol. 1995;69:285–287. doi: 10.1254/jjp.69.285. [DOI] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Dal-Zotto S, Marti O, Armario A. Glucocorticoids are involved in the long-term effects of a single immobilization stress on the hypothalamic-pituitary-adrenal axis. Psychoneuroendocrinology. 2003;28:992–1009. doi: 10.1016/s0306-4530(02)00120-8. [DOI] [PubMed] [Google Scholar]
- Dias Elpo Zomkowski A, Oscar Rosa A, Lin J, Santos AR, Calixto JB, Lucia Severo Rodrigues A. Evidence for serotonin receptor subtypes involvement in agmatine antidepressant like-effect in the mouse forced swimming test. Brain Res. 2004;1023:253–263. doi: 10.1016/j.brainres.2004.07.041. [DOI] [PubMed] [Google Scholar]
- Feng Y, Halaris AE, Piletz JE. Determination of agmatine in brain and plasma using high-performance liquid chromatography with fluorescence detection. J Chromatogr B Biomed Sci Appl. 1997;691:277–286. doi: 10.1016/s0378-4347(96)00458-6. [DOI] [PubMed] [Google Scholar]
- Feng Y, LeBlanc MH, Regunathan S. Agmatine reduces extracellular glutamate during pentylenetetrazole-induced seizures in rat brain: a potential mechanism for the anticonvulsive effects. Neurosci Lett. 2005;390:129–133. doi: 10.1016/j.neulet.2005.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea E, Regunathan S, Eliopoulos V, Feinstein DL, Reis DJ. Inhibition of mammalian nitric oxide synthases by agmatine, an endogenous polyamine formed by decarboxylation of arginine. Biochem J. 1996;316(Pt 1):247–249. doi: 10.1042/bj3160247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galea LA, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gilad GM, Gilad VH, Tizabi Y. Aging and stress-induced changes in choline and glutamate uptake in hippocampus and septum of two rat strains differing in longevity and reactivity to stressors. Int J Dev Neurosci. 1990;8:709–713. doi: 10.1016/0736-5748(90)90064-9. [DOI] [PubMed] [Google Scholar]
- Gilad GM, Salame K, Rabey JM, Gilad VH. Agmatine treatment is neuroprotective in rodent brain injury models. Life Sci. 1996;58:PL 41–46. doi: 10.1016/0024-3205(95)02274-0. [DOI] [PubMed] [Google Scholar]
- Iyo AH, Zhu MY, Ordway GA, Regunathan S. Expression of arginine decarboxylase in brain regions and neuronal cells. J Neurochem. 2006;96:1042–1050. doi: 10.1111/j.1471-4159.2005.03544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Yenari MA, Giffard RG, Cho SW, Park KA, Lee JE. Agmatine reduces infarct area in a mouse model of transient focal cerebral ischemia and protects cultured neurons from ischemia-like injury. Exp Neurol. 2004;189:122–130. doi: 10.1016/j.expneurol.2004.05.029. [DOI] [PubMed] [Google Scholar]
- Lavinsky D, Arteni NS, Netto CA. Agmatine induces anxiolysis in the elevated plus maze task in adult rats. Behav Brain Res. 2003;141:19–24. doi: 10.1016/s0166-4328(02)00326-1. [DOI] [PubMed] [Google Scholar]
- Li YF, Gong ZH, Cao JB, Wang HL, Luo ZP, Li J. Antidepressant-like effect of agmatine and its possible mechanism. Eur J Pharmacol. 2003;469:81–88. doi: 10.1016/s0014-2999(03)01735-7. [DOI] [PubMed] [Google Scholar]
- Lowy MT, Gault L, Yamamoto BK. Adrenalectomy attenuates stress-induced elevations in extracellular glutamate concentrations in the hippocampus. J Neurochem. 1993;61:1957–1960. doi: 10.1111/j.1471-4159.1993.tb09839.x. [DOI] [PubMed] [Google Scholar]
- Madrigal JL, Caso JR, de Cristobal J, Cardenas A, Leza JC, Lizasoain I, Lorenzo P, Moro MA. Effect of subacute and chronic immobilisation stress on the outcome of permanent focal cerebral ischaemia in rats. Brain Res. 2003;979:137–145. doi: 10.1016/s0006-8993(03)02892-0. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS. Stress-induced atrophy of apical dendrites of hippocampal CA3c neurons: involvement of glucocorticoid secretion and excitatory amino acid receptors. Neuroscience. 1995;69:89–98. doi: 10.1016/0306-4522(95)00259-l. [DOI] [PubMed] [Google Scholar]
- Magarinos AM, Deslandes A, McEwen BS. Effects of antidepressants and benzodiazepine treatments on the dendritic structure of CA3 pyramidal neurons after chronic stress. Eur J Pharmacol. 1999;371:113–122. doi: 10.1016/s0014-2999(99)00163-6. [DOI] [PubMed] [Google Scholar]
- Marquez C, Belda X, Armario A. Post-stress recovery of pituitary-adrenal hormones and glucose, but not the response during exposure to the stressor, is a marker of stress intensity in highly stressful situations. Brain Res. 2002;926:181–185. doi: 10.1016/s0006-8993(01)03112-2. [DOI] [PubMed] [Google Scholar]
- Marquez C, Nadal R, Armario A. The hypothalamic-pituitary-adrenal and glucose responses to daily repeated immobilisation stress in rats: individual differences. Neuroscience. 2004;123:601–612. doi: 10.1016/j.neuroscience.2003.10.016. [DOI] [PubMed] [Google Scholar]
- McIntosh LJ, Sapolsky RM. Glucocorticoids increase the accumulation of reactive oxygen species and enhance adriamycin-induced toxicity in neuronal culture. Exp Neurol. 1996;141:201–206. doi: 10.1006/exnr.1996.0154. [DOI] [PubMed] [Google Scholar]
- Minami M, Kuraishi Y, Yamaguchi T, Nakai S, Hirai Y, Satoh M. Immobilization stress induces interleukin-1 beta mRNA in the rat hypothalamus. Neurosci Lett. 1991;123:254–256. doi: 10.1016/0304-3940(91)90944-o. [DOI] [PubMed] [Google Scholar]
- Moghaddam B. Stress preferentially increases extraneuronal levels of excitatory amino acids in the prefrontal cortex: comparison to hippocampus and basal ganglia. J Neurochem. 1993;60:1650–1657. doi: 10.1111/j.1471-4159.1993.tb13387.x. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Bolinao ML, Stein-Behrens B, Sapolsky R. Glucocorticoids mediate the stress-induced extracellular accumulation of glutamate. Brain Res. 1994;655:251–254. doi: 10.1016/0006-8993(94)91622-5. [DOI] [PubMed] [Google Scholar]
- Nadeem A, Masood A, Masood N, Gilani RA, Shah ZA. Immobilization stress causes extra-cellular oxidant-antioxidant imbalance in rats: restoration by L-NAME and vitamin E. Eur Neuropsychopharmacol. 2006;16:260–267. doi: 10.1016/j.euroneuro.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Olivenza R, Moro MA, Lizasoain I, Lorenzo P, Fernandez AP, Rodrigo J, Bosca L, Leza JC. Chronic stress induces the expression of inducible nitric oxide synthase in rat brain cortex. J Neurochem. 2000;74:785–791. doi: 10.1046/j.1471-4159.2000.740785.x. [DOI] [PubMed] [Google Scholar]
- Otake K, Ruggiero DA, Regunathan S, Wang H, Milner TA, Reis DJ. Regional localization of agmatine in the rat brain: an immunocytochemical study. Brain Res. 1998;787:1–14. doi: 10.1016/s0006-8993(97)01200-6. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Elsevier; Oxford, UK: 2005. [DOI] [PubMed] [Google Scholar]
- Raasch W, Regunathan S, Li G, Reis DJ. Agmatine is widely and unequally distributed in rat organs. Ann N Y Acad Sci. 1995;763:330–334. doi: 10.1111/j.1749-6632.1995.tb32419.x. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Miller M, Janssen WG, Liston C, Hof PR, McEwen BS, Morrison JH. Repeated stress induces dendritic spine loss in the rat medial prefrontal cortex. Cereb Cortex. 2006;16:313–320. doi: 10.1093/cercor/bhi104. [DOI] [PubMed] [Google Scholar]
- Reagan LP, McEwen BS. Controversies surrounding glucocorticoid-mediated cell death in the hippocampus. J Chem Neuroanat. 1997;13:149–167. doi: 10.1016/s0891-0618(97)00031-8. [DOI] [PubMed] [Google Scholar]
- Regunathan S, Feinstein DL, Raasch W, Reis DJ. Agmatine (decarboxylated arginine) is synthesized and stored in astrocytes. Neuroreport. 1995;6:1897–1900. doi: 10.1097/00001756-199510020-00018. [DOI] [PubMed] [Google Scholar]
- Retana-Marquez S, Bonilla-Jaime H, Vazquez-Palacios G, Dominguez-Salazar E, Martinez-Garcia R, Velazquez-Moctezuma J. Body weight gain and diurnal differences of corticosterone changes in response to acute and chronic stress in rats. Psychoneuroendocrinology. 2003;28:207–227. doi: 10.1016/s0306-4530(02)00017-3. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM. Glucocorticoids, hippocampal damage and the glutamatergic synapse. Prog Brain Res. 1990;86:13–23. doi: 10.1016/s0079-6123(08)63163-5. [DOI] [PubMed] [Google Scholar]
- Sapolsky RM, Krey LC, McEwen BS. Prolonged glucocorticoid exposure reduces hippocampal neuron number: implications for aging. J Neurosci. 1985;5:1222–1227. doi: 10.1523/JNEUROSCI.05-05-01222.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shintani F, Nakaki T, Kanba S, Sato K, Yagi G, Shiozawa M, Aiso S, Kato R, Asai M. Involvement of interleukin-1 in immobilization stress-induced increase in plasma adrenocorticotropic hormone and in release of hypothalamic monoamines in the rat. J Neurosci. 1995;15:1961–1970. doi: 10.1523/JNEUROSCI.15-03-01961.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa N, Lukoyanov NV, Madeira MD, Almeida OF, Paula-Barbosa MM. Reorganization of the morphology of hippocampal neurites and synapses after stress-induced damage correlates with behavioral improvement. Neuroscience. 2000;97:253–266. doi: 10.1016/s0306-4522(00)00050-6. [DOI] [PubMed] [Google Scholar]
- Steciuk M, Kram M, Kramer GL, Petty F. Immobilization-induced glutamate efflux in medial prefrontal cortex: blockade by (+)-Mk-801, a selective NMDA receptor antagonist. Stress. 2000;3:195–199. doi: 10.3109/10253890009001123. [DOI] [PubMed] [Google Scholar]
- Stein-Behrens BA, Lin WJ, Sapolsky RM. Physiological elevations of glucocorticoids potentiate glutamate accumulation in the hippocampus. J Neurochem. 1994;63:596–602. doi: 10.1046/j.1471-4159.1994.63020596.x. [DOI] [PubMed] [Google Scholar]
- Sunanda Rao MS, Raju TR. Effect of chronic restraint stress on dendritic spines and excrescences of hippocampal CA3 pyramidal neurons--a quantitative study. Brain Res. 1995;694:312–317. doi: 10.1016/0006-8993(95)00822-8. [DOI] [PubMed] [Google Scholar]
- Tabor CW, Tabor H. Polyamines. Annu Rev Biochem. 1984;53:749–790. doi: 10.1146/annurev.bi.53.070184.003533. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Shankaranarayana Rao BS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang WP, Iyo AH, Miguel-Hidalgo J, Regunathan S, Zhu MY. Agmatine protects against cell damage induced by NMDA and glutamate in cultured hippocampal neurons. Brain Res. 2006;1084:210–216. doi: 10.1016/j.brainres.2006.02.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watanabe Y, Gould E, McEwen BS. Stress induces atrophy of apical dendrites of hippocampal CA3 pyramidal neurons. Brain Res. 1992;588:341–345. doi: 10.1016/0006-8993(92)91597-8. [DOI] [PubMed] [Google Scholar]
- Weng XC, Gai XD, Zheng JQ, Li J. Agmatine blocked voltage-gated calcium channel in cultured rat hippocampal neurons. Acta Pharmacol Sin. 2003;24:746–750. [PubMed] [Google Scholar]
- Yang XC, Reis DJ. Agmatine selectively blocks the N-methyl-D-aspartate subclass of glutamate receptor channels in rat hippocampal neurons. J Pharmacol Exp Ther. 1999;288:544–549. [PubMed] [Google Scholar]
- Zheng JQ, Weng XC, Gai XD, Li J, Xiao WB. Mechanism underlying blockade of voltage-gated calcium channels by agmatine in cultured rat hippocampal neurons. Acta Pharmacol Sin. 2004;25:281–285. [PubMed] [Google Scholar]
- Zhu MY, Wang WP, Bissette G. Neuroprotective effects of agmatine against cell damage caused by glucocorticoids in cultured rat hippocampal neurons. Neuroscience. 2006;141:2019–2027. doi: 10.1016/j.neuroscience.2006.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MY, Piletz JE, Halaris A, Regunathan S. Effect of agmatine against cell death induced by NMDA and glutamate in neurons and PC12 cells. Cell Mol Neurobiol. 2003;23:865–872. doi: 10.1023/A:1025069407173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MY, Wang WP, Huang J, Regunathan S. Chronic treatment with glucocorticoids alters rat hippocampal and prefrontal cortical morphology in parallel with endogenous agmatine and arginine decarboxylase levels. J Neurochem. 2007 doi: 10.1111/j.1471-4159.2007.04867.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu MY, Wang WP, Cai ZW, Regunathan S, Ordway G. Exogenous agmatine has neuroprotective effects against restraint-induced structural changes in the rat brain. Eur J Neurosci. 2008;27:1320–1332. doi: 10.1111/j.1460-9568.2008.06104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zomkowski AD, Hammes L, Lin J, Calixto JB, Santos AR, Rodrigues AL. Agmatine produces antidepressant-like effects in two models of depression in mice. Neuroreport. 2002;13:387–391. doi: 10.1097/00001756-200203250-00005. [DOI] [PubMed] [Google Scholar]


