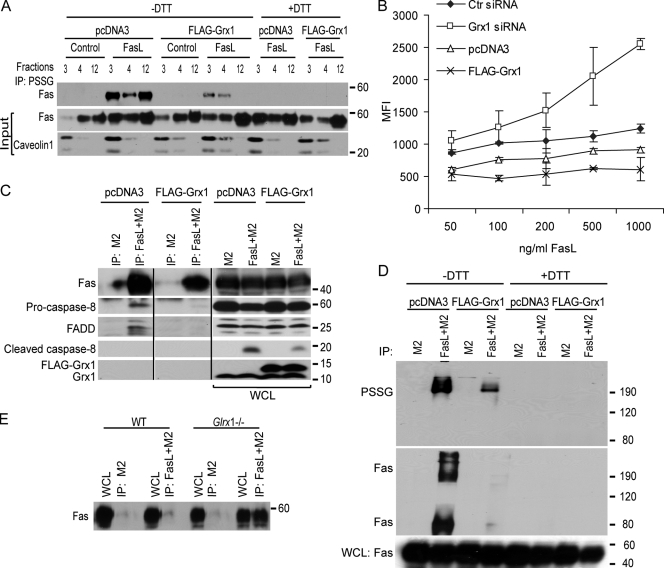
Figure 5.
Assessment of FasL binding, presence of Fas in lipid rafts, and DISC formation after manipulation of Grx1. (A) Evaluation of S-glutathionylation of Fas in lipid rafts in cells stimulated with FasL + M2 and the impact of overexpression of Grx1. Cells were transfected with pcDNA3 or Flag-Grx1 and stimulated with FasL + M2 for 20 min. Lipid raft fractions (3 and 4) and soluble fraction (12) were subjected to IP with antiglutathione antibody and analyzed by immunoblotting for Fas. PSSG was decomposed with 50 mM DTT as a reagent control before IP. The middle and bottom panels reflect immunoblot assays of Fas and the raft marker caveolin1 present in the input samples. Complete fractionation is shown in Fig. S3 A (available at http://www.jcb.org/cgi/content/full/jcb.200807019/DC1). (B) Assessment of FasL binding to cells after manipulation of Grx1. Cells were subjected to control (Ctr) and Grx1 siRNA transfection. In separate experiments, cells were transfected with pcDNA3 or Grx1 plasmids. After 48 h, cells were trypsinized and incubated with ascending doses of FasL + M2 for 20 min. Binding of FasL to cells was evaluated after incubation with FITC-conjugated anti–mouse antibody and evaluation of 10,000 events via flow cytometry. Binding of FasL to cells is reflected as mean fluorescence intensity (MFI), and absolute values are plotted on the y-axis. The x-axis depicts ascending concentrations of FasL. Note that differences absolute fluorescence intensities between pcDNA3 and control siRNA–transfected cells may be a result of the different transfection procedures. Confirmation of Grx1 overexpression and knockdown is shown in Fig. S3 B and Fig. S3 C, respectively. (C) Assessment of FasL-interacting proteins in cells overexpressing Grx1. pcDNA3 or Grx1-transfected C10 cells were treated with M2 alone or FasL + M2. Cells were lysed, and 700 µg of protein was subjected to IP using protein G agarose beads to isolate DISC proteins. After SDS-PAGE, samples were analyzed by immunoblotting for Fas, FADD, procaspase 8, cleaved caspase-8, and Grx1. IP, M2 represents control IP in the absence of FasL. Note that all samples were run on the same gel. Black lines indicate that intervening lanes have been spliced out. (D) Evaluation of PSSG and Fas content in high MW complexes after IP of FasL + M2 or M2 alone via nonreducing SDS PAGE. As a control, samples were treated with DTT before electrophoresis. (E) Assessment of interaction between FasL and Fas in WT primary tracheal epithelial cells or cells lacking Glrx1. Cells were exposed to M2 alone or FasL + M2 for 30 min, lysed, and 700 µg of protein was subjected to IP using protein G agarose beads. After SDS-PAGE, samples were analyzed by immunoblotting for Fas. WCL, whole cell lysate.