Abstract
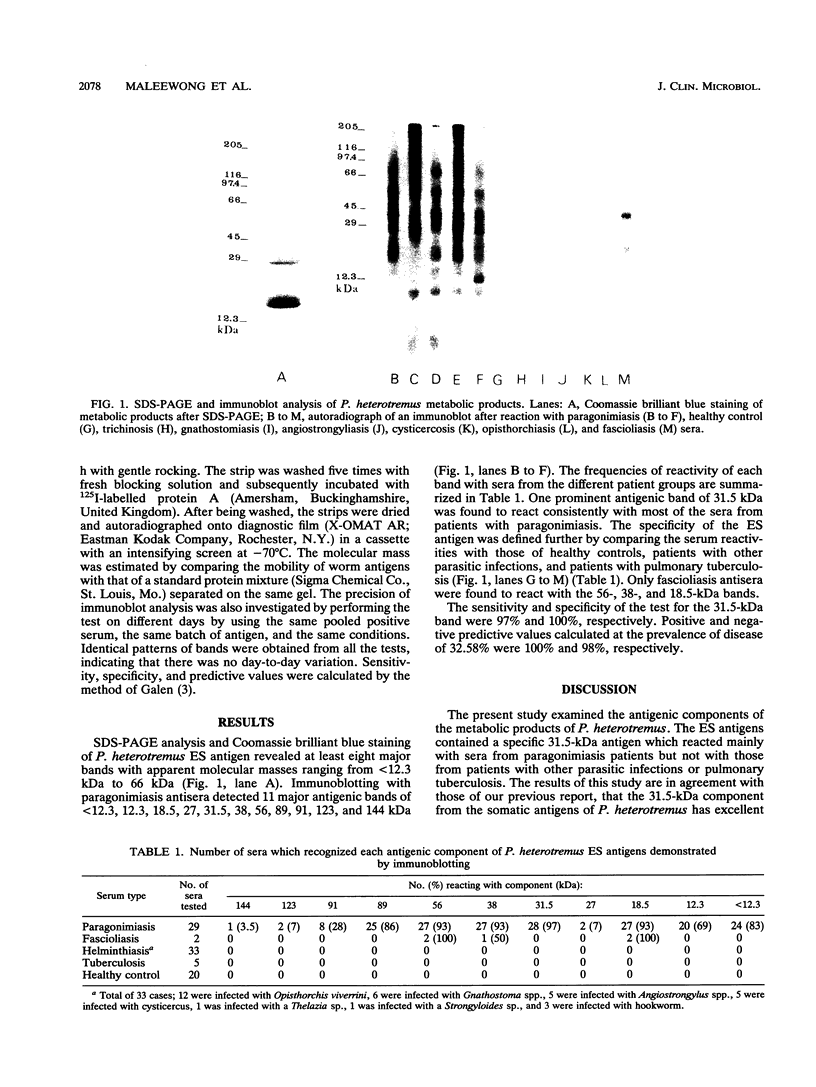
Antigenic components of Paragonimus heterotremus metabolic products were revealed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and immunoblot analysis of sera from patients with P. heterotremus infection, from patients with other illnesses, and from healthy adults. By SDS-PAGE, it was found that the metabolic products comprised more than eight major polypeptides. Immunoblot analysis revealed 11 components which were strongly recognized by paragonimiasis antisera. These antigenic components had molecular masses ranging from less than 12.3 kDa to 144 kDa. One antigenic band of 31.5 kDa was found to give a consistent reaction with paragonimiasis antisera (97% sensitivity). Of the other patient sera, only sera from patients with Fasciola sp. infection reacted with antigenic bands of 56, 38, and 18.5 kDa. The present findings suggest that the 31.5-kDa component is sensitive and specific for the diagnosis of human P. heterotremus paragonimiasis.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Erdman D. D. Clinical comparison of ethyl acetate and diethyl ether in the formalin-ether sedimentation technique. J Clin Microbiol. 1981 Nov;14(5):483–485. doi: 10.1128/jcm.14.5.483-485.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galen R. S. Predictive value and efficiency of laboratory testing. Pediatr Clin North Am. 1980 Nov;27(4):861–869. doi: 10.1016/s0031-3955(16)33930-x. [DOI] [PubMed] [Google Scholar]
- Indrawati I., Chaicumpa W., Setasuban P., Ruangkunaporn Y. Studies on immunodiagnosis of human paragonimiasis and specific antigen of Paragonimus heterotremus. Int J Parasitol. 1991 Jul;21(4):395–401. doi: 10.1016/0020-7519(91)90096-p. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Maleewong W., Pariyanonda S., Wongkham C., Intapan P., Daenseegaew W., Morakote N. Comparison of adult somatic and excretory-secretory antigens in enzyme-linked immunosorbent assay for serodiagnosis of human infection with Paragonimus heterotremus. Trans R Soc Trop Med Hyg. 1990 Nov-Dec;84(6):840–841. doi: 10.1016/0035-9203(90)90102-k. [DOI] [PubMed] [Google Scholar]
- Maleewong W., Wongkham C., Pariyanonda S., Intapan P., Pipitgool V., Daenseegaew W., Morakote N. Antigenic components of Paragonimus heterotremus recognized by infected human serum. Parasite Immunol. 1991 Jan;13(1):89–93. doi: 10.1111/j.1365-3024.1991.tb00265.x. [DOI] [PubMed] [Google Scholar]
- Pariyanonda S., Maleewong W., Pipitgool V., Wongkham C., Morakote N., Intapan P., Thirakharn B., Boonphadung N. Serodiagnosis of human paragonimiasis caused by Paragonimus heterotremus. Southeast Asian J Trop Med Public Health. 1990 Mar;21(1):103–107. [PubMed] [Google Scholar]
- Slemenda S. B., Maddison S. E., Jong E. C., Moore D. D. Diagnosis of paragonimiasis by immunoblot. Am J Trop Med Hyg. 1988 Nov;39(5):469–471. doi: 10.4269/ajtmh.1988.39.469. [DOI] [PubMed] [Google Scholar]
- Sugiyama H., Sugimoto M., Akasaka K., Horiuchi T., Tomimura T., Kozaki S. Characterization and localization of Paragonimus westermani antigen stimulating antibody formation in both the infected cat and rat. J Parasitol. 1987 Apr;73(2):363–367. [PubMed] [Google Scholar]
- Thongkrajai P., Lulitanon V., Chamnanvanakit C. Improved ELISA with immunoabsorbent-purified mycobacterial antigen for serodiagnosis of tuberculosis. J Med Microbiol. 1989 Oct;30(2):101–104. doi: 10.1099/00222615-30-2-101. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tuti S., Vichasri S., Sirisinha S. Effect of culture media on production of excretory-secretory products and egg output of Opisthorchis viverrini in vitro. J Parasitol. 1982 Oct;68(5):892–897. [PubMed] [Google Scholar]
- Vanijanonta S., Radomyos P., Bunnag D., Harinasuta T. Pulmonary paragonimiasis with expectoration of worms: a case report. Southeast Asian J Trop Med Public Health. 1981 Mar;12(1):104–106. [PubMed] [Google Scholar]



