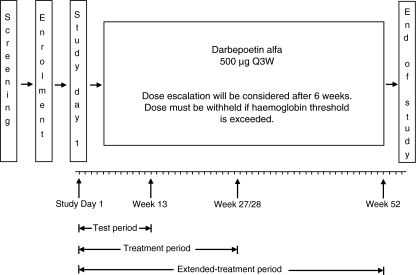
Fig 1.
Study schema. Darbepoetin alfa was administered at 500 μg every 3 weeks (Q3W). After 6 weeks (at week 7), the dosing frequency could be escalated to every 2 weeks (Q2W). Treatment was withheld when a patient's haemoglobin reached ≥130 g/l. Darbepoetin alfa treatment was not to exceed 52 weeks, and end of study was planned for 3 weeks (at week 55 for Q3W dosing) or 2 weeks (at week 53 for Q2W dosing) after the last dose of darbepoetin alfa was administered. Study day 1 (the first day of darbepoetin alfa administration) to week 13 was designated as ‘test period’, study day 1 to week 27/28 was designated as ‘treatment period’ and study day 1 to week 52 was designated as ‘extended-treatment period’.