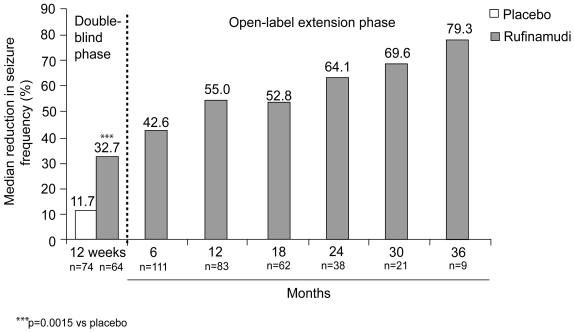
Figure 1.
Median percentage reduction from baseline in total seizure frequency during 12-week double-blind and subsequent open-label rufinamide treatment (Glauser 2005a, 2005b; Eisai, data on file). Data shown separately for patients receiving rufinamide (n=74) or placebo (n=64) during double-blind treatment (Glauser 2005a), and combined for patients continuing in the extension study and receiving open-label rufinamide treatment (n=124) (Glauser 2005b).