Figure 2.
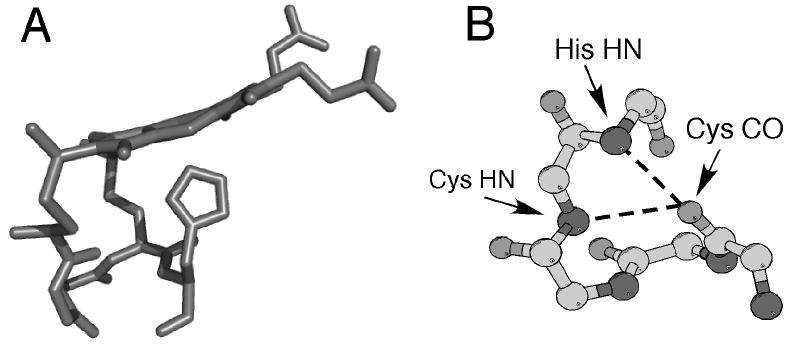
(A) Three-dimensional structure of heme c (side view) and the CXXCH (residues 14-18) pentapeptide from the structure of horse cytochrome c (PDB: 1HRC). The side chains of residues 15 and 16 are omitted for clarity. Note the nonplanar structure of the heme. (B) Ball-and-stick representation of backbone atoms of CXXCH motif. Hydrogen bonding interactions between the backbone HN of the second Cys and the His to the backbone CO of the first His are shown with dashed lines.
