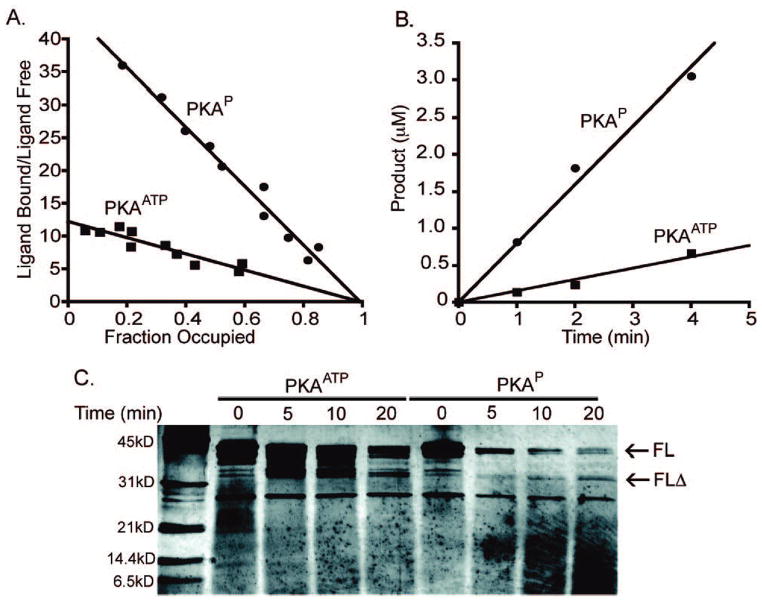
Figure 3.
Comparative properties of PKAATP and PKAP. (A) Fluorescence binding analysis of semisynthetic PKA. PKAATP or PKAP (1 μM) was incubated with and without various concentrations of Mant-ADP (5–125 μM) in the presence of 1 M free Mn. Scatchard analysis revealed Mant-ADP showed a Kdapp of 77 ± 20 μM for PKAATP and 20 ± 2 μM for PKAP. (B) Kinase activities of semisynthetic PKAs. Radiometric assays were carried out using 5 nM enzyme, 30 μM biotinylated kemptide substrate, 10 μM ATP, and 10 mM MgCl2. Turnover numbers were found to be 36 ± 8 min−1 for PKAATP and 160 ± 10 min−1 for PKAP. (C) Limited proteolysis (trypsin) of PKAATP and PKAP using 1:5 trypsin/PKA at 4 °C for the period of time indicated. Silver-stained SDSPAGE revealed a metastable fragment (FLΔ) from PKAATP and N-terminal sequence revealed cleavage after Lys83 for FLΔ.