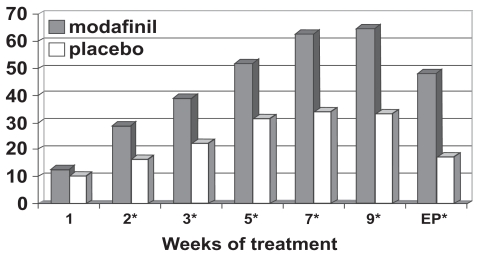
Figure 4b.
Percentage responders as a function of time for a flexible-dose study of efficacy of modafinil for children and adolescents with attention-deficit/hyperactivity disorder (n = 244). Response is defined as having a Clinical Global Impressions of Change score of 1 (“very much improved”) or 2 (“much improved”). *p values <0.05; EP = endpoint, which represents the last obtained value carried forward. Reproduced from Cephalon, Inc. 2006. Modafinil (CEP-1538) tablets Supplemental NDA 20-717/S-019 ADHD indication. Briefing document for Psychopharmacologic Drugs Advisory Committee Meeting March 26, 2006. Frazer, PA: Cephalon, Inc.