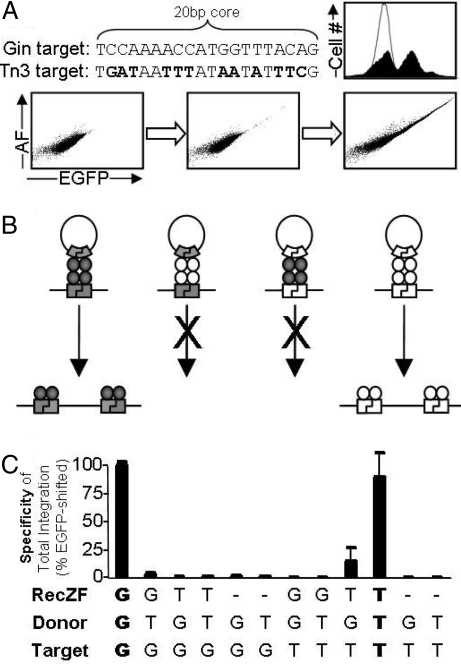
Fig. 6.
Study of the contribution of catalytic domain specificity to RecZF target specificity. Novel RecZFs can be generated by combining hyperactive serine catalytic domains (Table S1) and zinc finger proteins. Tn3C5 was generated by fusing a hyperactive catalytic domain of the Tn3 resolvase to the C5 zinc finger protein. (A) Like GinC5, Tn3C5 catalyzed highly specific plasmid integration. (B) Illustration of RecZF target site selectivity. RecZF specificity is a product of both zinc finger recognition and catalytic domain sequence dependence. Because the Gin and Tn3 catalytic domains are specific for different core sequences (20G and 20T, respectively; Table 1), RecZFs derived from these 2 elements are functionally orthogonal. (C) Selectivity of the Gin and Tn3 catalytic domains was assayed under all possible combinations of RecZF (GinC5, G; Tn3C5, T), donor (C.20G-Puro, G; C.20T-Puro, T), and genomic target (293-C.20G, G; 293-C.20T, T). High levels of specific recombination were only observed when enzyme matched both substrates (shown in bold). Standard error is shown for samples prepared in triplicate.