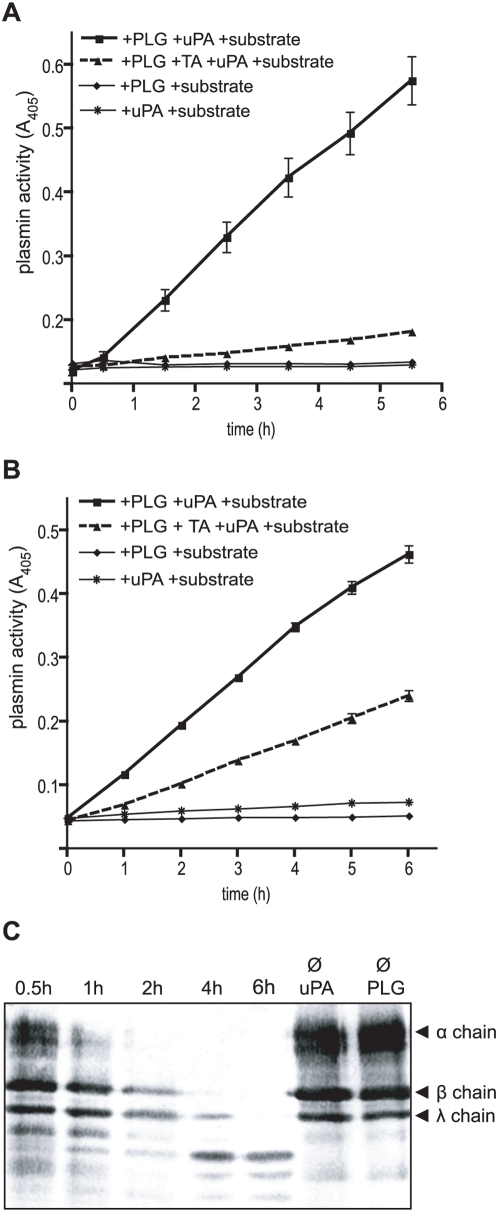
Figure 7. Activation and proteolytic activity of HcpA- and B. recurrentis-bound plasmin(ogen).
Intact B. recurrentis organisms (A) or recombinant HcpA (B) were incubated with PLG. Bound PLG was converted into plasmin by uPA addition and plasmin activity was measured using the chromogenic substrate D-Val-Leu-Lys 4-nitroanilide dihydrochloride (S-2251). uPA mediated PLG activation was inhibited by tranexamic acid (TA). Substrate cleaving was monitored by measurement of the absorbance at 405 nm for up to 6 hrs. Mean of triplicates ± SEM is shown. (C) Degradation of fibrinogen by HcpA-bound plasmin. HcpA coated microtiter plates were incubated with PLG, subsequently fibrinogen and uPA were added. The reaction mixtures were separated by SDS-PAGE, transferred to nitrocellulose and probed with rabbit anti-fibrinogen followed by peroxidase-conjugated IgG for detection of α, β and γ chains (67, 57 and 47 kDa) and the small-size degradation products of fibrinogen.