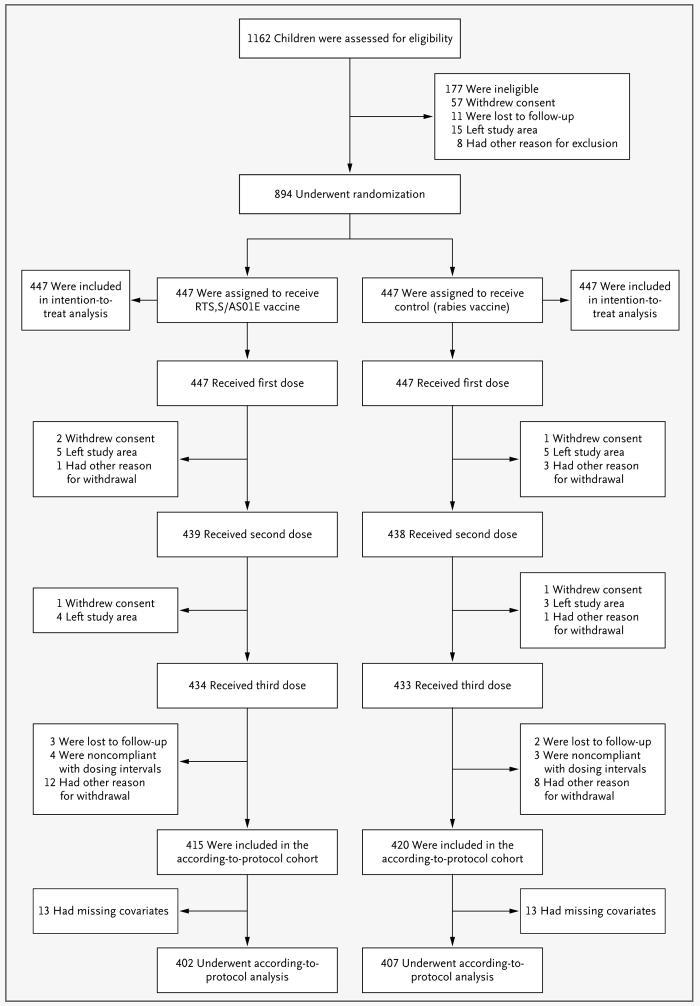
Figure 1. Screening, Randomization, and Follow-up of Study Participants.
The 177 children who were deemed ineligible at screening had an age outside the acceptable range (67 children), an acute disease at enrollment (23), a serious illness on clinical screening (42), a laboratory result outside the acceptable limit (28), or another reason for ineligibility: a parent or guardian who was judged by the investigator to be unable to give consent, an inability to follow the protocol, a planned administration of another vaccination, or the use of blood products in the previous 3 months (17). The other reasons for ineligibility or withdrawal from the study were enrollment in other clinical trials (for the eight children excluded at screening only), missed vaccinations because of hospital admission, contraindications to further vaccination, medical conditions not permitted according to the protocol, and unavailability of documentation about concomitant vaccination.