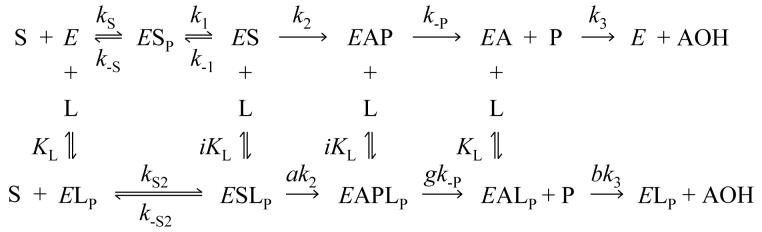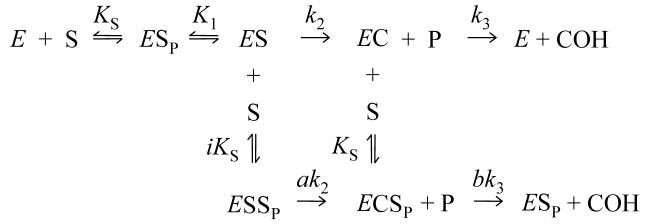Abstract
Acetylcholinesterase (AChE) contains a narrow and deep active site gorge with two sites of ligand binding, an acylation site (or A-site) at the base of the gorge and a peripheral site (or P-site) near the gorge entrance. The P-site contributes to catalytic efficiency by transiently binding substrates on their way to the acylation site, where a short-lived acylated enzyme intermediate is produced. Carbamates are very poor substrates that, like other AChE substrates, form an initial enzyme-substrate complex with free AChE (E) and proceed to an acylated enzyme intermediate (EC) which is then hydrolyzed. However, the hydrolysis of EC is slow enough to resolve the acylation and deacylation steps on the catalytic pathway. Here we focus on the reaction of carbachol (carbamoylcholine) with AChE. The kinetics and thermodynamics of this reaction are of special interest because carbachol is an isosteric analog of the physiological substrate acetylcholine. We show that the reaction can be monitored with thioflavin T as a fluorescent reporter group. The fluorescence of thioflavin T is strongly enhanced when it binds to the P-site of AChE, and this fluorescence is partially quenched when a second ligand binds to the A-site to form a ternary complex. Analysis of the fluorescence reaction profiles was challenging, because four thermodynamic parameters and two fluorescence coefficients were fitted from the combined data both for E and for EC. Respective equilibrium dissociation constants of 6 and 26 mM were obtained for carbachol binding to the A- and P-sites in E and of 2 and 32 mM for carbachol binding to the A- and P-sites in EC. These constants for the binding of carbachol to the P-site are about an order of magnitude larger (i.e., indicating lower affinity) than previous estimates for the binding of acetylthiocholine to the P-site.
Keywords: Acetylcholinesterase, thioflavin T, carbamoylation, peripheral site, enzyme mechanism
Acetylcholinesterase (AChE)1 (FOOTNOTE 1) hydrolyzes the neurotransmitter acetylcholine at one of the highest known enzymatic rates (5). Interest in AChE inhibitors has been stimulated by observations that a number of these inhibitors, including carbamoyl esters, have been shown to have therapeutic benefits. In particular, AChE inhibitors that penetrate the blood-brain barrier elevate the levels of endogenous acetylcholine and are useful in the symptomatic treatment of Alzheimer's disease (6).
A better understanding of the AChE catalytic pathway may identify new mechanisms of AChE inhibition. An essential feature of AChE activity at many cholinergic synapses is the ability to hydrolyze acetylcholine within a millisecond of its release (7). Insights into this high catalytic efficiency were obtained from ligand binding studies (1, 8, 9) and X-ray crystallography (10, 11), which revealed a narrow active site gorge some 20 Å deep with two separate ligand binding sites. At the base of the gorge is the acylation or A-site where residue W86 2 (FOOTNOTE 2) binds the trimethylammonium group of acetylcholine and H447, E334, and S203 participate in a triad that catalyzes the transient acylation and deacylation of S203 during each substrate turnover. The peripheral or P-site, spanned by residues W286 near the mouth of the gorge and D74 near a constriction at the boundary between the P-site and the A-site, specifically binds certain ligands like the neurotoxin fasciculin (12, 13) and the fluorescent probes propidium (9) and thioflavin T (1).
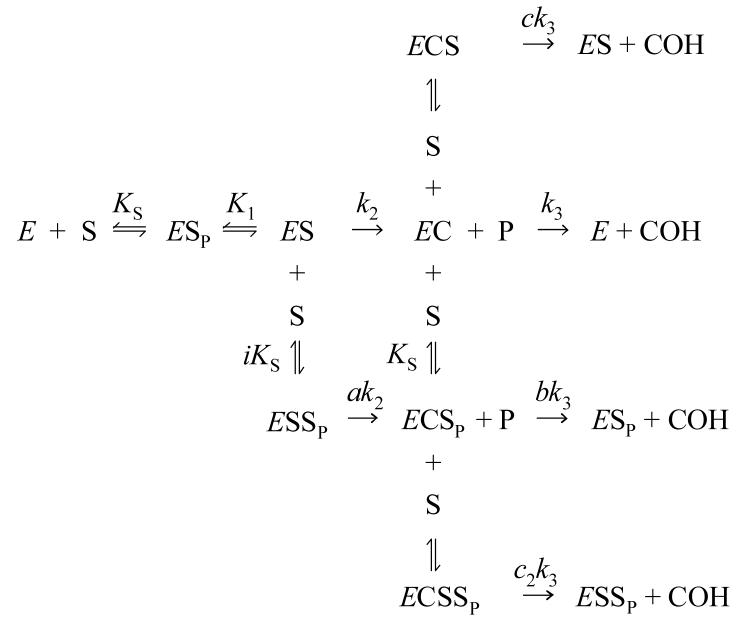
The translation of these structural insights into a functional understanding of the AChE catalytic mechanism is challenging. A series of previous reports from our laboratory indicates that Scheme 1 provides a minimal mechanistic framework that can account for most catalytic features of AChE (2, 4, 14-17).
SCHEME 1.
Scheme 1 includes an initial complex with substrate S bound to the P-site (ESP, where the subscript P denotes the ligand bound to the P-site) and a subsequent complex with substrate shifted to the A-site (ES) where S203 is acylated to generate EAP. P in this complex is the initial product that remains after transfer of the acyl group, and the catalytic pathway concludes by dissociation of P and hydrolysis of the acylated enzyme EA to give the second product AOH. Ternary complexes in which intermediates also bind a ligand L at the P-site (ESLP, EAPLP,and EALP) allow the rate constants for conversion of one intermediate to the next to be altered by the relative factors a, g, or b, respectively. The ligand L may be a second molecule of substrate or an inhibitor with affinity for the P-site. The formation of these ternary complexes can account for substrate hydrolysis profiles that deviate from a classical Michaelis-Menten kinetic formulation. We have reported that substrate inhibition with acetylthiocholine (a decrease in hydrolysis rates v at high S concentration) results from g ≅ 0 in the EAPSP complex (15); that substrate activation with the cationic acetanilide ATMA (an increase in v at moderate S concentration) arises from a ≅ > 1 in the ESSP complex (2); and that inhibition by P-site inhibitors (I) results in part from low values of kS2 in the EIP complex (14). Others have contended that substrate activation results from b ≅ > 1 in the EASP complex (18). One difficulty in resolving these sometimes conflicting claims is that the rate equation for v that is derived from Scheme 1 has too many unknown kinetic parameters, and a number of very different sets of parameter values fit the data equally well (2, 19). Furthermore, some of the reversible reactions in Scheme 1 are not at equilibrium, and in this case v in general can be solved only by numerical integration (2, 17). This can add a few more unknown kinetic parameters. Progress can be made, however, by grouping parts of Scheme 1 that are in equilibrium, by choosing substrates that eliminate parts of Scheme 1, and by determining some of the thermodynamic parameters in Scheme 1 by experiments that are independent of measurements of v. All three of these approaches are incorporated in this report.
The class of AChE inhibitors comprised of carbamoyl esters (here referred to as carbamates) are actually very poor substrates for AChE. Like other AChE substrates, they form an initial enzyme-substrate complex ES and proceed to an acylated enzyme intermediate which is then hydrolyzed. However, unlike carboxylic acid ester substrates, the hydrolysis of the carbamoylated enzyme is slow enough to resolve the acylation and deacylation steps. Despite this striking advantage offered by carbamates, few comprehensive analyses of their reactions with AChE have been undertaken. Here we report on the interaction of AChE with carbachol, a carbamate whose reaction mechanism with AChE is particularly important because it is an isosteric analog of acetylcholine (Figure 1). We show that the reaction of carbachol with AChE can be monitored with thioflavin T (Figure 1) as a fluorescent reporter group. The fluorescence of thioflavin T is strongly enhanced when it binds to the P-site of AChE, and this fluorescence is partially quenched when a second ligand binds to the A-site to form a ternary complex (1). These fluorescence changes allow the determination of carbachol affinities for the A- and P-sites both in the free enzyme and in the carbamoylated enzyme intermediate.
Figure 1.
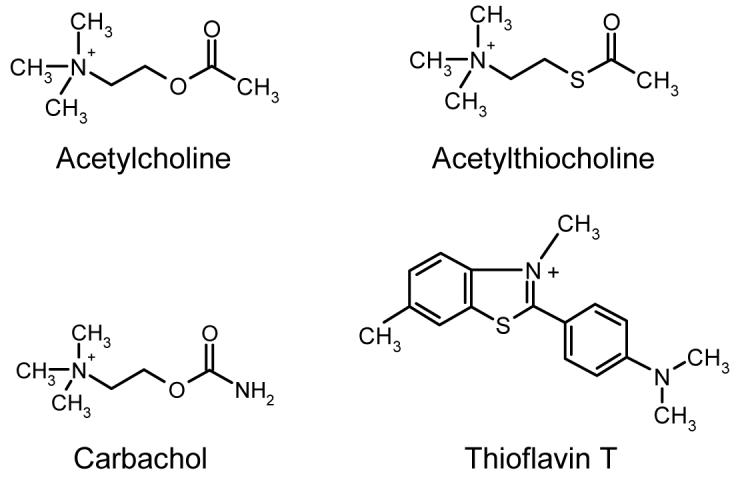
Structures of acetylcholine analogs and thioflavin T.
Experimental Methods
Materials
Recombinant human AChE was expressed as a secreted, disulfide-linked dimer in Drosophila S2 cells and purified as outlined previously (16). Total AChE concentrations (Etot) were calculated assuming 450 units/nmol (1).3 FOOTNOTE 3. Thioflavin T and carbachol (carbamoylcholine chloride) were from Sigma. Thioflavin T was recrystallized from water, and concentrations were assigned by absorbance at 412 nm with ε412 nm = 36,000 M−1cm−1 (with water as the solvent).
Fluorescence titration of AChE with thioflavin T
The fluorescence was monitored on a Perkin-Elmer LS-50B luminescence spectrometer thermostatted at 25 °C. Fluorescence excitation was at 450 nm and emission at 490 nm, with excitation and emission slits of 10 to 20 nm. Mixing thioflavin T and AChE in a thermostatted Hi-Tech SFA 20 stopped flow apparatus gave a stable fluorescence signal (F) over a 3 - 4 min recording interval. Average F values were corrected for inner filter effects (20) as described previously (2). Data were consistent with a single thioflavin T binding site on AChE and analyzed with eq 1, a slight extension of a previous equation (1).
| (1) |
In eq 1, D = [E]tot + [L]tot + KL, where [E]tot and [L]tot are the total enzyme (E) and thioflavin T (L) concentrations, respectively, and KL is the equilibrium dissociation constant; FB = B + fQ[L]tot + fE[E]tot, where B is the blank fluorescence without E or L and fQ = fL/(1 + ([L]tot/KQ)); and fL, fEL and fE are the fluorescence intensity coefficients for free thioflavin T, bound thioflavin T, and free enzyme, respectively. Values of fL, fE, B and KQ were determined from measurements with thioflavin T or enzyme alone. The parameter KQ was introduced here to account for slight self-quenching at high concentrations of thioflavin T. Data were fitted to eq 1 by unweighted nonlinear regression analysis (Fig.P version 6.0c), with [L]tot as the independent variable and KL and fEL as the fitted parameters.
Stopped-Flow Measurements of Carbachol Reaction with AChE
Stopped-flow methods were required to accurately measure the approach to the carbamoylation steady state with thioflavin T as a fluorescent reporter ligand. The thermostatted Hi-Tech SFA 20 stopped flow apparatus described above was used to rapidly mix equal volumes (300 ul) of AChE in one syringe and carbachol in the other. Fluorescence measurements were obtained as described above and recorded at fixed intervals as short as 20 msec. Thioflavin T was added at equal concentrations to both syringes in 40 mM sodium phosphate, 0.04% Triton X-100 at pH 7.0. To maintain constant ionic strength when the range of carbachol concentrations extended to 60 mM, additional NaCl was included added so that the sum of the carbachol and NaCl concentrations was 60 mM.
A framework for kinetic analysis of AChE carbamoylation
With carbamate substrates, both the formation and hydrolysis of the acylated enzyme intermediate, or carbamoyl enzyme (EC), are slow enough to allow equilibrium assumptions. A general approach is given in Scheme 2 (4, 21).
SCHEME 2.
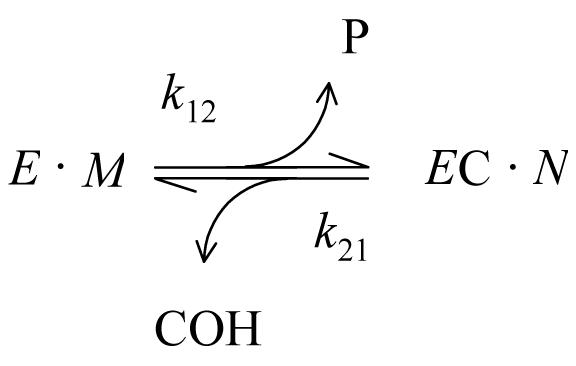
In this scheme, k12 is an overall carbamoylation rate constant that incorporates the intrinsic rate constant k2 from Scheme 1, and k21 is an overall decarbamoylation rate constant that incorporates k3. P and COH are the products released during the carbamoylation and decarbamoylation reactions, respectively. The terms E•M and EC•N represent all species in equilibrium with E and EC, respectively (i.e., [E]•M + [EC]•N = [E]tot, where [E] and [EC] are the respective concentrations of E and EC and Etot is the total concentration of enzyme active sites), and M and N are sums of terms (involving rate and equilibrium constants and ligand concentrations) that depend on the details of a selected reaction scheme. The rate equation corresponding to Scheme 2 can be solved explicitly if all ligand concentrations remain essentially constant over the reaction time course (21, 22), as recently described (4). When [EC] = 0 at time t = 0, the solutions are given in eqs 2 and 3.
| (2) |
| (3) |
Determination of carbachol affinities for the AChE A- and P-sites with thioflavin T as a fluorescent reporter
Thioflavin T fluorescence was employed to evaluate the interactions of carbachol at the A- and P-sites during the carbamoylation reaction. In the context of Scheme 2, one expression for the time dependence of F is given by eq 4. In eq. 4, F0 is the fluorescence at time = 0 and FF is the fluorescence when carbamoylation has reached a final steady state.
| (4) |
F also can be formulated in the more useful terms FEM and FECN, the respective fluorescence contributions from species equilibrated with E and EC, as shown in eq 5.
| (5) |
In eq. 5, FB = B + fL[L] + fS[S], where B is the blank fluorescence with E in the absence of L or S and the molar fluorescence coefficients fL and fS are determined from measurements with thioflavin T or carbachol alone. Combining eqs. 2, 3, and 5 gives eq. 6.
| (6) |
While eq 6 provided a good fit to the fluorescence data, we routinely applied a slightly extended version of this equation (eq A2) that permitted incorporation of a slower component of relatively small amplitude (see the Appendix).
Because k21 is much smaller than k12 for the reaction of virtually all carbamates with AChE, the exponential time course involving conversion of initial E to predominant EC in the final steady state is readily apparent with thioflavin T as the fluorescent reporter. However, k12 and k21 are not resolved individually in this fluorescence analysis. Although k21 is small, it is not completely negligible relative to k12, and the individual values of these rate constants do slightly influence the fitted values of FECN. These rate constants are resolved when the carbachol reaction is monitored by simultaneous hydrolysis of the reporter substrate acetylthiocholine (4, 23), and we have inserted predetermined values of k21 (which ranged from 0.03 to 0.16 min−1) for each carbachol concentration (4) as a fixed parameter in fitting to eq 6 and A2. Although k12 and k21 contain important mechanistic information (e.g., (4)), their analysis is not undertaken in this report in order to focus on the useful thermodynamic information obtained by measuring FEM and FECN over a range of ligand concentrations.
Expressions for k12, k21, M and N are derived by selecting a particular reaction scheme. One model for the reaction of carbamate substrates with AChE is presented in Scheme 3 (4).
SCHEME 3.
This scheme is simplified from Scheme 1 because k2 and k3 with carbamates represent slow reactions that eliminate EAP as a significant intermediate and allow all ligand binding to reach equilibrium. The acylated enzyme intermediate EA in Scheme 1 becomes the carbamoylated enzyme EC in Scheme 3. Since our analysis here assumes no EC at time t = 0, the relevant species from Scheme 3 and the new species with thioflavin T bound to the P-site at time t = 0 are given in Scheme 4.
SCHEME 4.
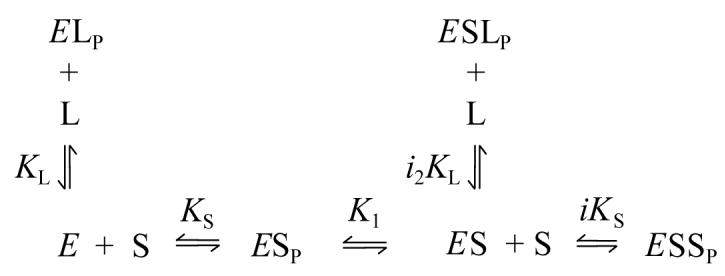
In Scheme 4 the affinity of thioflavin T (L) at the P-site (denoted by subscript P) is characterized by the dissociation constant KL in the absence of carbachol (S) and by i2KL when S occupies the A-site. When all reactions in Scheme 4 are assumed to reach equilibrium (e.g., [E][L] = KL[ELP], etc.), M from Scheme 2 is given by eq. 7.
| (7) |
In eq 7 KM is equal to KSK1 and KM+S is given in eq. 8.
| (8) |
Scheme 4 can be linked with Scheme 2 by noting that the only fluorescent complexes in Scheme 4 are ELP and ESLP. The total fluorescence from these complexes is fEL[ELP] + fESL[ESLP], where the coefficients fEL and fESL denote the molar fluorescence of thioflavin T in the binary complex ELP and the ternary complex ESLP, respectively. Therefore, combining this expression with eqs. 4 and 6 leads to eqs 9 and 10.
| (9) |
| (10) |
Eq. 10 is a slightly rewritten form of a previous equation (2), and it assumes that [L] ≅ [L]tot and [S] ≅ [S]tot. FEM data were initially fitted to eq. 10 by nonlinear regression analysis (SigmaPlot version 10) with [S] as the independent variable, [L], KL, i and i2 as fixed parameters, and KM, iKS, fEL and fEL/fESL as the fitted parameters. The value of KL at the ionic strength employed here was 1.91 μM (23). The only fitted parameter retained from these analyses was fEL, which remained constant at a given [L] over a wide range of the input i and i2 values. Control reactions with AChE and thioflavin T alone were interspersed with runs that included carbachol to confirm the stability of fEL. A second cycle of data fitting was then conducted in which FEM/fEL from multiple data sets, each at different fixed [L], were analyzed simultaneously with eq 10 in the numerical integration program SCoP (Simulation Resources, Inc., Redlands, CA; version 3.52) as described previously (1). In these simultaneous fits either i or i2 became one of the four fitted parameters. In addition, KM was replaced with KM+S as one of the fitted parameters with the substitution KM = KM+SiKS/(iKS − (i)(KM+S)) (from eq. 8).
Results and Discussion
The fluorescence of thioflavin T is enhanced by a factor of more than 800 when it binds to AChE (1, 23). The fluorescence increase is consistent with thioflavin T interaction at a single site in human AChE with an equilibrium dissociation constant of 1 - 2 μM (23). The structure of the thioflavin T complex with TcAChE obtained by X-ray crystallography (24) indicates that the ligand binds to the AChE P-site, with the benzothiazole ring of thioflavin T stacked against Trp279 and its dimethylaminophenyl moiety 3.5Å distant from and nearly coplanar with the phenyl group of Phe330. The two rings of thioflavin T are planar, and this loss of rotational mobility is thought to provide the fluorescence enhancement in thioflavin T-AChE complexes (see (25). The partial quenching of this fluorescence when an A-site ligand binds to form a ternary complex can also be explained by three-dimensional structures. In crystal structures of TcAChE with two ligands specific for the A-site, edrophonium (26) and TMTFA (11), the phenyl ring of Phe330 is rotated ∼115° relative to its position in the thioflavin T-TcAChE complex. In this orientation, the phenyl ring would partially overlap with the crystal structure location of thioflavin T. Therefore, the proximity of the phenyl ring is likely to slightly distort the planarity of thioflavin T and decrease the fluorescence in the ternary complex.
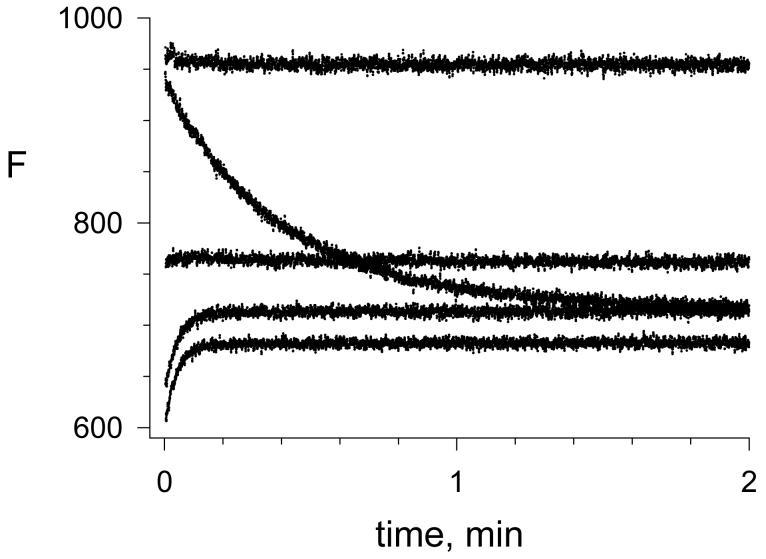
The difference in thioflavin T fluorescence intensity between these binary and ternary complexes allows the binding of both A and P-site ligands to be quantified (1, 2). Fluorescence changes when carbachol was rapidly mixed with the binary complex of thioflavin T and AChE depended on the carbachol concentration (Figure 2). With 0.5 mM carbachol in Figure 2, the fluorescence decreased about 25% over the 2-minute time course in which AChE was largely converted to the carbamoylated enzyme EC. At a higher 10 mM concentration of carbachol, almost no fluorescence change was observed during the reaction, and at 40 and 60 mM carbachol rapid 10 - 15% increases in fluorescence were observed (Figure 2). Thioflavin T can bind to both E and EC, and the direction of the fluorescence change depended on the relative quenching of complexes formed by E and EC. The kinetics of the fluorescence change corresponded closely to a single exponential reaction, although a slightly better fit was obtained when a second, minor exponential component was included (Figure 3 and see the Appendix).
Figure 2.
Reactions of carbachol with AChE monitored by thioflavin T fluorescence. Reactions were initiated by stopped-flow mixing of AChE with carbachol and thioflavin T as outlined in the Experimental Procedures, and the fluorescence F was recorded continuously. Initial concentrations of thioflavin T (15.6 μM) and AChE (152 nM) were the same in each reaction, while the respective concentrations of carbachol were 0, 0.5, 10, 40, and 60 mM (from the upper to the lower trace taken at time t = 0). FB (eq 5) from the components individually was 83.
Figure 3.
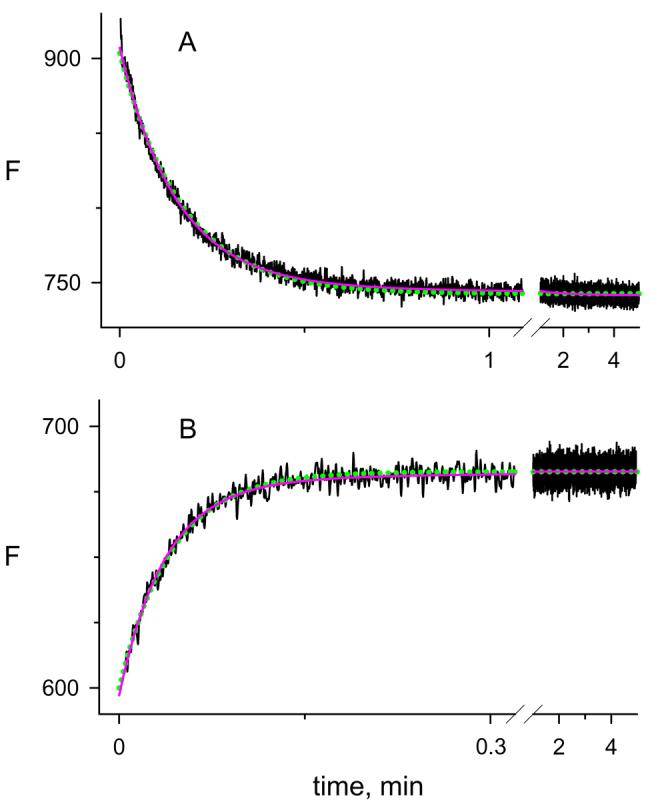
Analysis of a reaction of carbachol with AChE. Reaction traces were obtained in the presence of thioflavin T under the conditions in Figure 2 with 1.67 mM carbachol in Panel A or 60 mM carbachol in Panel B. Traces were analyzed in SigmaPlot both with the one-component, single-exponential fit in eq 6 and the two-component, double exponential fit in eq A2 in the Appendix to obtain values of FEM, FECN, and k12 + k21. The solid red line shows the two-component fit and the dotted green line indicates the one-component fit. Respective values of k12 + k21 for the overall one- and two-component fits were 5.94 ± 0.02 and 6.53 ± 0.02 min−1 in Panel A and 23.8 ± 0.3 and 27.0 ± 0.4 min−1 in Panel B. In the two-component fits, k122 = 0.68 ± 0.04 in Panel A and 4.3 ± 0.3 in Panel B.
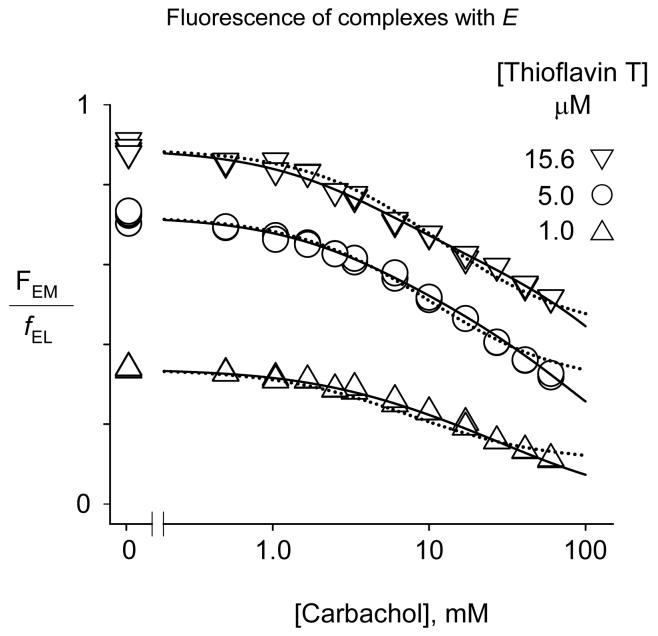
To obtain equilibrium binding constants for carbachol with the free enzyme E, we focused on the initial fluorescence F0 prior to the formation of EC in reaction traces like those in Figure 2. F0 (or FEM, which is (F0 - FB) normalized by [E]tot; see eq 6) decreased progressively with increasing carbachol concentration, consistent with the expected partial quenching of thioflavin T fluorescence in the reversible complex formed when carbachol binds to the A-site in the ternary complex involving E. However, analysis of the FEM data is challenging. Scheme 4 includes four independent equilibrium constants for carbachol binding (KM, KS, i2KM, and iKS) and two fluorescence coefficients (fEL and fESL), and it is difficult for an FEM analysis to uniquely assign all six parameters. FEM was obtained as a function of the carbachol concentration from traces like those in Figure 2 in three experiments, each at a different fixed concentration of thioflavin T, and FEM/fEL from these data sets were then fitted simultaneously to eq. 10 as outlined in Figure 4. We previously showed that the equilibrium constants corresponding to KM and i2 for the binding of the specific A-site ligand edrophonium in its binary complex with E and its ternary complex with E and thioflavin T could be assigned unambiguously from a similar combination of fluorescence data sets (1). However, edrophonium does not show significant binding to the P-site (equivalent to eliminating ESSP in Scheme 4 by setting iKS−1 = 0 in eqs 7 and 10). A similar one-site analysis could be applied to the carbachol data (dotted lines in Figure 4), but a better fit was obtained by including iKS in a two-site analysis. The higher affinity site was occupied by carbachol in the ternary complex with thioflavin T and AChE, and we assume that this is the A-site. The lower affinity site involved competition between carbachol and thioflavin T, and we assume this to be the P-site. The values of some of the fitted parameters, however, had a small dependence on the fixed value of i or i2 (Table 1). The two parameters independent of i or i2 were fEL/fESL and KM+S, a measure of the combined binding of carbachol to the A- and P-sites (see eq. 8). Ranges for the other fitted parameters are shown in the last two rows of Table 1. These ranges are dictated by the requirements that i be positive (fixing i2 at a maximum of 1.08) and that iKS > KM (fixing i2 at a minimum of 0.42). These are very conservative requirements. Based on previous estimates of i2 for thioflavin T with edrophonium (1.12, (1) and ATMA (3.1, (2), it is unlikely that either i or i2 with carbachol is much less than 1.0. A reasonable best estimate is to fix i2 at 0.90, which leads to the fitted parameters in the top row of Table 1 and suggests that the probable range of KM, iKS and i2 is within 20% of their values in this row. Slightly more uncertainty surrounds i, as its value is determined by the level of ESP, a very minor component in these titrations. However, it is likely to lie between 1 and a maximal value of 1.67, obtained when i2 was fixed at 0.54. Additional studies are required to determine whether even less ambiguity in these values can be achieved with simultaneous fitting of a greater number of data sets at different fixed concentrations of thioflavin T.
Figure 4.
Determination of carbachol binding constants with free AChE from the initial fluorescence of AChE-bound thioflavin T. From reference (23). FEM values from mixtures of AChE (70 - 155 nM), thioflavin T (1.0 - 15.6 μM, as noted), and the indicated concentration of carbachol were determined from stopped-flow traces as in Figure 3. The FEM data from each set at fixed thioflavin T concentration were fitted initially to eq 10 to obtain fEL for the set, and FEM/fEL from all three sets were then analyzed simultaneously with eq 10 as described in the Experimental Methods. Parameters obtained from the data fitting are shown in Table 1. The dotted lines show the one-site fit when carbachol is assumed to have no affinity for the P-site ((iKS)−1 fixed at 0) with fitted parameters KM+S = 6.2, fEL/fESL = 1.85, and i2 =2.1.
Table 1.
Thermodynamic constants for carbachol binding to free AChE (E) from the thioflavin T fluorescence coefficients FEM/fEL.
| i2 | i |
KM+S mM |
KM mM |
iKs mM |
fEL/fESL | |
|---|---|---|---|---|---|---|
| a | 0.90 fixed |
0.92 | 4.7 ± 0.4 | 5.7 ± 0.4 | 26 ± 4 | 1.50 ± 0.04 |
| b | 1.08 | 1e-6 fixed |
4.72 | 4.72 | 32 | 1.50 |
| b | 0.42 | 1.59 | 4.72 | 12 | 12 fixed |
1.50 |
Parameters from the simultaneous fit of the three data sets in Figure 4. Errors (SEM) were obtained by reanalyzing each data set individually with i fixed at 0.92, i2 fixed at 0.90, and KM+S, iKS, fEL and fEL/fESL as the fitted parameters. Fitted parameter values from the three individual sets were then averaged and SEM was determined.
Simultaneous fits of the three data sets in Figure 5 with fixed values selected to show maximum parameter range (see text).
One noteworthy comparison arising from Table 1 involves the parameter fEL/fESL. a measure of the relative quenching of thioflavin T fluorescence in the ternary complex involving free E. Its value of 1.50 in Table 1 and was significantly smaller than previous determinations of fEL/fESL with edrophonium (2.76 ± 0.02, (1) or ATMA (3.0 ± 0.2, (2). We contend above that this relative quenching arises from rotation of the phenyl ring of Phe330 in the ternary complex. The lower value of fEL/fESL for carbachol therefore implies that carbachol binding to the A-site induces a smaller rotation of the phenyl ring of Phe330 and less distortion of the planarity of thioflavin T in the ternary complex. This explanation is also consistent with the best estimates of 0.9 – 1.0 for i and i2 in Table 1. These values indicate no loss of affinity for the relatively small carbachol molecule in ternary complexes relative to binary complexes, in contrast to higher i2 values previously observed for ternary complexes involving larger cationic ligands (3.1 with ATMA and thioflavin T and 9.5 for ATMA and propidium (2). Since carbachol is an isosteric analog of acetylcholine, there is also likely to be little unfavorable steric or electrostatic interaction between bound acetylcholine molecules in AChE ternary complexes.
Information about carbachol binding to the carbamoylated enzyme EC is also available from reaction traces like those in Figure 2 through analysis of FECN (eq 5). While FECN makes the predominant contribution to the final steady-state fluorescence, FECN was actually determined by fitting the entire reaction time course to eq A2. Values of FECN/fECL in Figure 5 first increased slightly and then decreased with increasing carbachol concentration in each of the three data sets, a biphasic pattern that requires two carbachol binding sites in EC. The higher affinity site again involved carbachol binding in the ternary complex, and the most likely candidate is the A-site. There has long been indirect evidence from kinetic analyses that cationic inhibitors can bind to the A-site in acylated AChE even with the site partially occupied by the acyl group (see (5)), and a recent crystallographic study of acetylthiocholine with TcAChE indicated that the P- and A-sites can each be occupied by an acetylthiocholine molecule in the acetylated enzyme (27). Scheme 3 may be extended to allow substrate to bind to the A- and P-sites in the carbamoylated enzyme EC as shown in Scheme 5.
Figure 5.
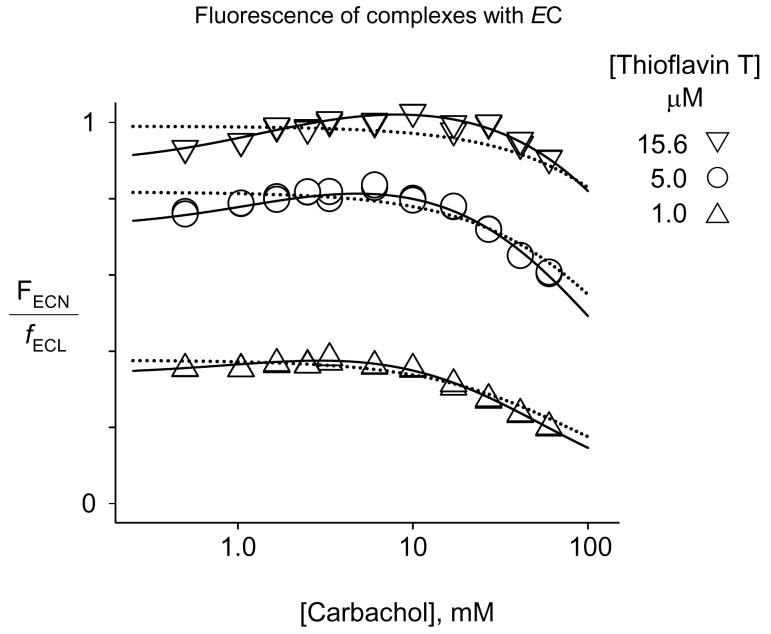
Determination of carbachol binding constants with carbamoylated AChE from the fluorescence of AChE-bound thioflavin T after the carbachol reactions have reached a final steady state. FECN values from mixtures of AChE (70 - 155 nM), thioflavin T (1.0 - 15.6 μM, as noted), and the indicated concentration of carbachol were determined from stopped-flow traces as in Figure 3. The FECN and then FECN/fECL data were analyzed with eq 11 as described for FEM and then FEM/fEL in Figure 4. Parameters obtained from the data fitting are shown in Table 2. The dotted lines show the fit when carbachol is assumed to bind to only one site, (e.g., carbachol and thioflavin T compete for the P-site: (iECKECS)−1 and iEC2−1 fixed at 0 with fitted KM+S = 57).
SCHEME 5.
One group has in fact attributed one component of substrate inhibition to a block of deacylation when the substrate binds to the A-site in the acylated enzyme (c = c2 = 0 in Scheme 5; (18)), although other interpretations of the kinetic data are possible. In a parallel version of Scheme 4, E is replaced by EC in all of the proposed enzyme species and the subscripts for all of the kinetic and thermodynamic parameters are prefaced by EC. The resulting expression for FECN is given in eq 11.
| (11) |
FECN/fECL values from data sets at each of the three thioflavin T concentrations were fitted simultaneously to eq. 11 as outlined in Figure 5, and the fitted parameters are shown in Table 2. The format of this table is identical to that in Table 1, and a similar analysis of the range of fitted parameters as that presented for Table 1 above revealed the same pattern of dependence on the fixed values of iEC or iEC2. The overall consistency of the FEM and FECN data is highlighted by the agreement in the affinities of carbachol for the P-site in E (iKS of 26 mM) and in EC (iECKECS of 32 mM) and in the small difference in carbachol affinities between binary and ternary complexes (best estimates of i, i2, iEC, and iEC2 are all close to 1). In contrast to the consistent quenching of thioflavin T fluorescence in ternary complexes with carbachol and E noted above, the fitted value of fECL/fECSL (0.83) indicated that the fluorescence of bound thioflavin T actually increased about 20% when carbachol bound to the A-site in the ternary complex with EC. This increase made the determinations of KECM+S and KECM possible, but its small magnitude resulted in larger relative errors for these parameters than those for KM+S and KM in Table 1. Furthermore, the KECM+S of 1.7 mM was nearly three-fold lower than KM+S, indicating that ECSP was very minor relative to ECS and making estimation of iEC even more difficult than that of i. A very conservative estimate is that iEC is likely to lie between 1 and a maximal value of 5.2, obtained when iEC2 was fixed at 0.48.
Table 2.
Thermodynamic constants for carbachol binding to carbamoylated AChE (EC) from the thioflavin T fluorescence coefficients FECN/fECL.
| iEC2 | iEC |
KECM+S mM |
KECM mM |
iECKECS mM |
fECL/fECSL | |
|---|---|---|---|---|---|---|
| a | 0.90 fixed |
1.23 | 1.7 ± 0.4 | 1.8 ± 0.5 | 32 ± 2 | 0.83 ± 0.01 |
| b | 0.96 | 1e-6 fixed |
1.66 | 1.66 | 34 | 0.83 |
| b | 0.21 | 3.5 | 1.66 | 8 | 8 fixed |
0.83 |
Analyses of FECN were conducted with eq 11 and the data in Figure 5 following the fitting procedures described for FEM in footnotes a and b in Table 1. One measurement of KECL = 1.82 ± 0.26 μM in the presence of 2 mM carbachol agreed with the previously determined KL (1.91 ± 0.18 μM from four experiments; (23)), and KECL was also fixed at 1.91 μM in all analyses. Thermodynamic parameters correspond to a modified Scheme 4 in which E is replaced by EC and all subscripts are preceded by EC. Errors (SEM) were obtained by reanalyzing each data set individually with iEC fixed at 1.23 and iEC2 fixed at 0.90.
Calculation of the parameter fECSL/fESL (1.14 ± 0.03 for the three sets in Tables 1 and 2, not all data shown) indicates that thioflavin T fluorescence was actually less quenched when carbachol bound to the A-site in the ternary complex with the carbamoylated enzyme ECSLP than with the free enzyme ESLP. This suggests less steric interaction between bound thioflavin T and bound carbachol in ECSLP than in ESLP. Therefore, if the location of carbachol binding in the A-site is slightly different in E and EC, the somewhat higher affinity of carbachol for the A-site in EC can be rationalized.
An important application of the values determined for the thermodynamic parameters in Tables 1 and 2 is their comparison with independent measurements of some of the same parameters from analyses of k12 and k21 for carbachol with AChE. These comparisons can be used to support and refine reaction schemes for the AChE catalytic pathway. For example, a preliminary analysis of k12 with carbachol did not clearly reveal a second carbachol binding site and thus could not assign values to iKS, i or i2, even with the higher precision obtained with acetylthiocholine as the reporter substrate rather than thioflavin T. However, this analysis did estimate KM+S to be 2.8 ± 0.2 mM (4), about 60% of the KM+S value in Table 1. A parallel preliminary analysis of k21 assigned a KECM of 9 ± 1 mM based on the decrease in k21 with increasing carbachol concentration (4), a value 5-fold higher than KECM in Table 2. Despite the uncertainties in the measurement of KECM here as noted above, this difference appears to be beyond the variance of the measurements and suggests that a revised kinetic model for the previous analysis of k21 may be necessary.
Because carbachol is an isosteric analog of acetylcholine and acetylthiocholine, differences in their affinities for the A- and P-sites are likely to reflect their distinctive electronic distributions around the carbonyl carbon atom. The affinities of acetylcholine and acetylthiocholine for the A-site have not been measured, and the likelihood that binding does not reach equilibrium (15, 28) will probably prevent their measurement. In contrast to analyses of substrates in equilibrated systems, KM with these substrates is a combination of rate constants and cannot be used as a measure of affinity. One measurement of acetylthiocholine affinity for the P-site has been made and is based on inhibition of rates of fasciculin association with the P-site. The reported value of about 1 mM (15) was obtained at a somewhat lower ionic strength than employed here, but even compensating conservatively for this difference the 26 and 32 mM values of iKS and iECKECS for the binding of carbachol to the P-site in Tables 1 and 2 are an order of magnitude higher than that expected for iKS with acetylthiocholine. Acetylthiocholine appears to interact with W286, F337, Y124, Y72 (27, 29) and indirectly with D74 (17) in the P-site, and further studies are necessary to determine the precise interactions that increase its affinity by an order of magnitude over carbachol in this site.
Appendix
Carbamate reactions with AChE are fitted fairly well with the single exponential formulation in Scheme 2 and eqs 1 and 2, but slightly better fits are obtained if a minor second exponential term is added (T. L. Rosenberry et al., manuscript in preparation). We assumed that the second minor exponential corresponded to carbachol reactions with a small amount of AChE (E2) with slightly different catalytic properties from those of the major AChE component E1. A similar small component was observed previously during measurements of organophosphate reactions with our recombinant human AChE, and we attributed it to a minor AChE form (16), perhaps differing in the size or location of glycosyl groups. The corresponding extension of eq 5 is given in eq A1, where 2 denotes terms involving the minor E2 component.
| (A1) |
The E2 component was only slightly evident with the carbachol reactions monitored by thioflavin T fluorescence here (see Figure 3), but it was much clearer with the fluorogenic carbamate M7C and with carbachol reactions monitored with acetylthiocholine as a reporter substrate (4), where values of R = [E2]tot/[E1]tot of 0.02 to 0.10 were obtained (T. L. Rosenberry et al., manuscript in preparation). Here, R was fixed at 0.05. The intent in including E2 in analyzing carbamate reactions was simply to improve the accuracy of values assigned to the major component E1, and no analysis of reactions involving E2 was attempted. The precision of our measurements was sufficient to indicate only that the corresponding k122 for E2 was 3- to 10-fold lower than the k12 for E1. In the absence of evidence of other differences between the major and minor reaction components, k21, FEM, and FECN were assumed to be the same for both components. Eq 6 then is extended to eq A2.
| eq. (A2) |
Footnotes
This work was supported by grant NS-16577 from the National Institutes of Health and by grants from the Muscular Dystrophy Association of America
Abbreviations: AChE, acetylcholinesterase; ATMA, 3-(acetamido)-N,N,N-trimethylanilinium; DTNB, 5,5′-dithiobis-(2-nitrobenzoic acid); M7C, 1-methyl-(7-dimethylcarbamoxy)quinolinium; TcAChE, Torpedo californica AChE; TMTFA, m-(N,N,N-trimethylammonio)trifluoroacetophenone.
Throughout this paper we number amino acid residues according to the human AChE sequence (Uniprot accession number P22303).
One unit of AChE activity corresponds to 1 μmol of acetylthiocholine hydrolyzed/min under standard pH-stat assay conditions at pH 8 (1). Our conventional spectrophotometric assay at 412 nm (2) is conducted in pH 7 buffer. With recombinant human AChE and 0.5 mM acetylthiocholine, this assay results in 4.8 ΔA412 nm/min with 1 nM AChE or about 76 % of the pH stat assay standard.
References
- 1.De Ferrari GV, Mallender WD, Inestrosa NC, Rosenberry TL. Thioflavin T is a fluorescent probe of the acetylcholinesterase peripheral site that reveals conformational interactions between the peripheral and acylation sites. J. Biol. Chem. 2001;276:23282–23287. doi: 10.1074/jbc.M009596200. [DOI] [PubMed] [Google Scholar]
- 2.Johnson JL, Cusack B, Davies MP, Fauq A, Rosenberry TL. Unmasking tandem site interaction in human acetylcholinesterase. Substrate activation with a cationic acetanilide substrate. Biochemistry. 2003;42:5438–5452. doi: 10.1021/bi027065u. [DOI] [PubMed] [Google Scholar]
- 3.Brufani M, Lippa S, Marta M, Oradei A, Pomponi M. Acetylcholinesterase inhibition by eserine: rate constants of reaction. Part II. Ital. J. Biochem. 1985;34:328–340. [PubMed] [Google Scholar]
- 4.Rosenberry TL, Johnson JL, Cusack B, Thomas J, Emani S, Venkatasubban KS. Interactions between the peripheral site and the acylation site in acetylcholinesterase. Chem. Biol. Interact. 2005;157-158:181–189. doi: 10.1016/j.cbi.2005.10.027. [DOI] [PubMed] [Google Scholar]
- 5.Rosenberry TL. Acetylcholinesterase. In: Meister A, editor. Advances in Enzymology. John Wiley & Sons; New York: 1975. pp. 103–218. [DOI] [PubMed] [Google Scholar]
- 6.Giacobini E. Cholinesterase inhibitors: from the Calabar bean to Alzheimer therapy. In: Giacobini E, editor. Cholinesterases and Cholinesterase Inhibitors. Martin Dunitz; London: 2000. pp. 181–226. [Google Scholar]
- 7.Rosenberry TL. Quantitative simulation of endplate currents at neuro-muscular junctions based on the reactions of acetylcholine with acetylcholine receptor and acetylcholinesterase. Biophys. J. 1979;26:263–290. doi: 10.1016/S0006-3495(79)85249-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Changeux J-P. Responses of acetylcholinesterase from Torpedo marmorata to salts and curarizing drugs. Mol. Pharmacol. 1966;2:369–392. [PubMed] [Google Scholar]
- 9.Taylor P, Lappi S. Interaction of fluorescence probes with acetylcholinesterase. The site and specificity of propidium binding. Biochemistry. 1975;14:1989–1997. doi: 10.1021/bi00680a029. [DOI] [PubMed] [Google Scholar]
- 10.Sussman JL, Harel M, Frolow F, Oefner C, Goldman A, Toker L, Silman I. Atomic structure of acetylcholinesterase from Torpedo californica: A prototypic acetylcholine-binding protein. Science. 1991;253:872–879. doi: 10.1126/science.1678899. [DOI] [PubMed] [Google Scholar]
- 11.Harel M, Quinn DM, Nair HK, Silman I, Sussman JL. The X-ray structure of a transition state analog complex reveals the molecular origins of the catalytic power and substrate specificity of acetylcholinesterase. J. Am. Chem. Soc. 1996;118:2340–2346. [Google Scholar]
- 12.Harel M, Kleywegt GJ, Ravelli RBG, Silman I, Sussman JL. Crystal structure of an acetylcholinesterase-fasciculin complex: interaction of a three-fingered toxin from snake venom with its target. Structure. 1995;3:1355–1366. doi: 10.1016/s0969-2126(01)00273-8. [DOI] [PubMed] [Google Scholar]
- 13.Bourne Y, Taylor P, Marchot P. Acetylcholinesterase inhibition by fasciculin: Crystal structure of the complex. Cell. 1995;83:503–512. doi: 10.1016/0092-8674(95)90128-0. [DOI] [PubMed] [Google Scholar]
- 14.Szegletes T, Mallender WD, Rosenberry TL. Nonequilibrium analysis alters the mechanistic interpretation of inhibition of acetylcholinesterase by peripheral site ligands. Biochemistry. 1998;37:4206–4216. doi: 10.1021/bi972158a. [DOI] [PubMed] [Google Scholar]
- 15.Szegletes T, Mallender WD, Thomas PJ, Rosenberry TL. Substrate binding to the peripheral site of acetylcholinesterase initiates enzymatic catalysis. Substrate inhibition arises as a secondary effect. Biochemistry. 1999;38:122–133. doi: 10.1021/bi9813577. [DOI] [PubMed] [Google Scholar]
- 16.Mallender WD, Szegletes T, Rosenberry TL. Organophosphorylation of acetylcholinesterase in the presence of peripheral site ligands: Distinct effects of propidium and fasciculin. J. Biol. Chem. 1999;274:8491–8499. doi: 10.1074/jbc.274.13.8491. [DOI] [PubMed] [Google Scholar]
- 17.Mallender WD, Szegletes T, Rosenberry TL. Acetylthiocholine binds to Asp74 at the peripheral site of human acetylcholinesterase as the first step in the catalytic pathway. Biochemistry. 2000;39:7753–7763. doi: 10.1021/bi000210o. [DOI] [PubMed] [Google Scholar]
- 18.Stojan J, Brochier L, Alies C, Colletier JP, Fournier D. Inhibition of Drosophila melanogaster acetylcholinesterase by high concentrations of substrate. Eur. J. Biochem. 2004;271:1364–1371. doi: 10.1111/j.1432-1033.2004.04048.x. [DOI] [PubMed] [Google Scholar]
- 19.Stojan J, Golicnik M, Fournier D. Rational polynomial equation as an unbiased approach for the kinetic studies of Drosophila melanogaster acetylcholinesterase reaction mechanism. Biochim. Biophys. Acta. 2004;1703:53–61. doi: 10.1016/j.bbapap.2004.09.017. [DOI] [PubMed] [Google Scholar]
- 20.Lakowicz JR. Principles of Fluorescence Spectroscopy. 2nd ed. Kluwer Academic/Plenum; New York: 1999. [Google Scholar]
- 21.Rosenberry TL, Bernhard SA. Studies of catalysis by acetylcholinesterase: Fluorescent titration with a carbamoylating agent. Biochemistry. 1971;10:4114–4120. doi: 10.1021/bi00798a016. [DOI] [PubMed] [Google Scholar]
- 22.Rosenberry TL, Bernhard SA. Studies on catalysis by acetylcholinesterase: Synergistic effects of inhibitors during hydrolysis of acetic acid esters. Biochemistry. 1972;11:4308–4321. doi: 10.1021/bi00773a018. [DOI] [PubMed] [Google Scholar]
- 23.Rosenberry TL, Sonoda LK, Dekat SE, Cusack B, Johnson JL. Monitoring the reaction of carbachol with acetylcholinesterase by thioflavin T fluorescence and acetylthiocholine hydrolysis. Chem.-Biol. Interact. 2008 doi: 10.1016/j.cbi.2008.06.002. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Harel M, Sonoda LK, Silman I, Sussman JL, Rosenberry TL. The crystal structure of thioflavin T bound to the peripheral site of Torpedo californica acetylcholinesterase reveals how thioflavin T acts as a sensitive fluorescent reporter of ligand binding to the acylation site. J. Am. Chem. Soc. 2008 doi: 10.1021/ja7109822. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Friedhoff P, Schneider A, Mandelkow EM, Mandelkow E. Rapid assembly of Alzheimer-like paired helical filaments from microtubule-associated protein tau monitored by fluorescence in solution. Biochemistry. 1998;37:10223–10230. doi: 10.1021/bi980537d. [DOI] [PubMed] [Google Scholar]
- 26.Harel M, Schalk I, Ehret-Sabatier L, Bouet F, Goeldner M, Hirth C, Axelsen PH, Silman I, Sussman JL. Quaternary ligand binding to aromatic residues in the active-site gorge of acetylcholinesterase. Proc. Natl. Acad. Sci. USA. 1993;90:9031–9035. doi: 10.1073/pnas.90.19.9031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Colletier JP, Fournier D, Greenblatt HM, Stojan J, Sussman JL, Zaccai G, Silman I, Weik M. Structural insights into substrate traffic and inhibition in acetylcholinesterase. EMBO J. 2006;25:2746–2756. doi: 10.1038/sj.emboj.7601175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rosenberry TL. Catalysis by acetylcholinesterase. Evidence that the rate-limiting step for acylation with certain substrates precedes general acid-base catalysis. Proc. Nat. Acad. Sci. USA. 1975;72:3834–3838. doi: 10.1073/pnas.72.10.3834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bourne Y, Radic Z, Sulzenbacher G, Kim E, Taylor P, Marchot P. Substrate and product trafficking through the active center gorge of acetylcholinesterase analyzed by crystallography and equilibrium binding. J. Biol. Chem. 2006;281:29256–29267. doi: 10.1074/jbc.M603018200. [DOI] [PubMed] [Google Scholar]