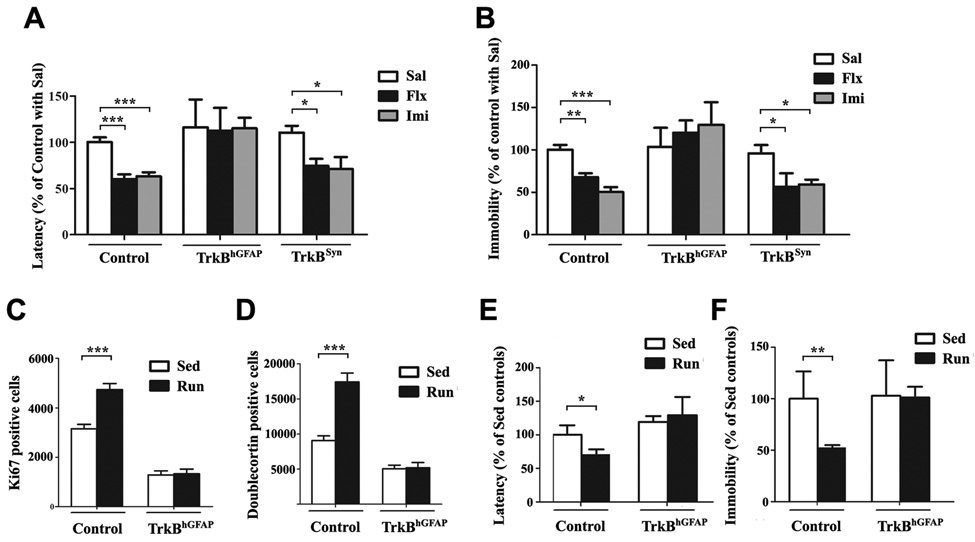
Figure 6. TrkBhGFAP mice were insensitive to chronic AD and exercise induced improvement in depression and anxiety-like behaviors.
(A) In the novelty-suppressed feeding test (NSFT), chronic treatments with fluoxetine or imipramine shortened the latency to feed (indicating reduced anxiety) in control, TrkBSyn but not TrkBhGFAP mice. Data were shown as percentage of control with saline injection. N=6–29 for each group. ANOVA (GLM) found significant effects of AD treatment (F2,100=8.022, p=0.0006) and genotype (F2,100=10.49, p<0.0001).
(B) The tail-suspension test (TST) measured total duration of immobility (“behavior despair”), which could be reduced by chronic fluoxetine or imipramine in control, TrkBSyn but not TrkBhGFAP mice. Data were shown as percentage of control with saline injection. N=7–28 for each. ANOVA (GLM) revealed significant effects of AD treatment (F2,100=4.233, p=0.0172), genotype (F2,100=20.03, p<0.0001) and the interaction of both (F2,100=5.085, p=0.0009).
(C–D) Running for 6 weeks failed to increase the number of Ki67 (C) or Doublecortin (D) positive cells in the DG of TrkBhGFAP mice. N=6–13 for each. ANOVA (GLM) revealed significant effects of running (F1,36=13.64, p=0.0007 for Ki67; F1,36=16.01, p=0.0003 for Doublecortin), genotype (F1,36=141.1, p<0.0001 for Ki67; F1,36=58.46, p<0.0001 for Doublecortin) and the interaction of the two (F1,36=12.66, p=0.0010 for Ki67; F1,36=14.79, p=0.0005 for Doublecortin).
(E–F) TrkBhGFAP mice did not display decrease in latency to feed (E, NSFT) or duration of immobility (F, TST) after 6 weeks of running, compared to sedentary controls. Data were shown as percentage of sedentary control. N=8–16 for each. NSFT (F1,41=15.09, p=0.0004 for genotype), TST (F1,41=9.082, p=0.0044 for genotype; F1,41=8.273, p=0.0064 for exercise).
*p<0.05; **p<0.01; ***p<0.001.