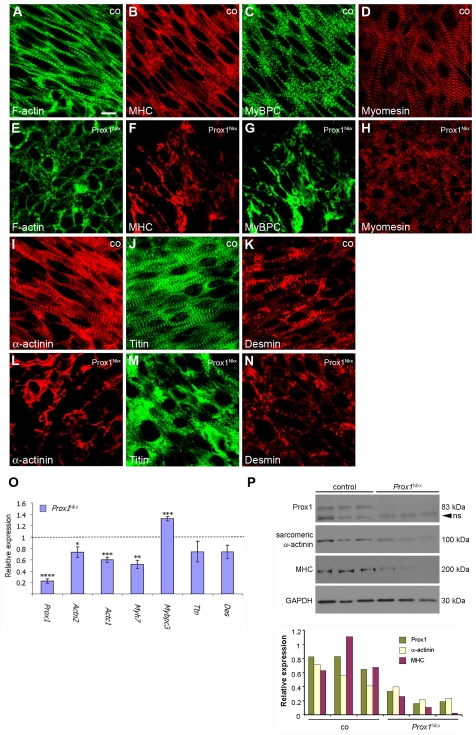
Fig. 3.
All structural components of the sarcomere are severely disrupted in Prox1-conditional myocardium. (A-N) Confocal sections of immunostained E13.5 whole-mount hearts from control (co; A-D,I-K) and Prox1Nkx (E-H,L-N) mouse embryos. Actin thin filaments and myosin thick filaments lack organisation and are not striated in Prox1-conditional hearts, as visualised by phalloidin staining (green; compare E with A) and immunostaining for sarcomeric myosin heavy chain (MHC) (red; B,F), respectively. Immunostaining for the thick filament component sarcomeric and cardiac myosin binding protein C (MyBP-C) further demonstrates thick filament disorganisation (green; C,G). M-band disruption is demonstrated by immunostaining for myomesin (red; D,H). Z-disc disruption in Prox1Nkx hearts is revealed by immunostaining for sarcomeric α-actinin (red; I,L), titin N-terminus (green; J,M) and desmin (red; K,N). (O) Quantitative real-time PCR (qRT-PCR) for sarcomere component genes on E12.5 Prox1Nkx hearts. Data are presented as mean ± s.e.m.; *P<0.05, **P<0.003, ***P<0.001, ****P<9×10-7. (P) Western blots of E13.5 control and Prox1Nkx individual (half) heart lysates for Prox1 [non-specific (ns) band indicated by arrowhead], sarcomeric α-actinin, sarcomeric MHC and Gapdh, and quantification of protein levels, as normalised to Gapdh, using scanning densitometry. Scale bar: 10 μm.