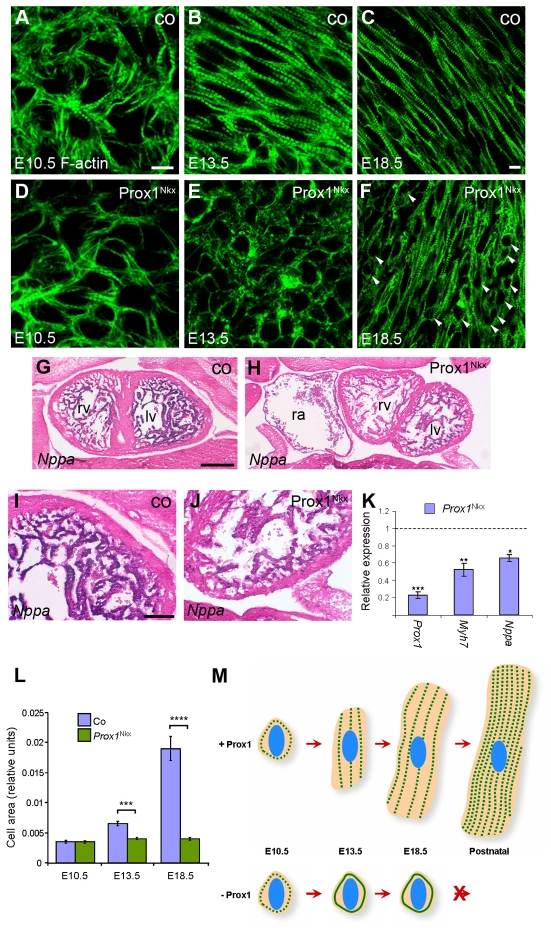
Fig. 5.
Prox1 is required for foetal cardiomyocyte hypertrophy. (A-F) Phalloidin staining on E10.5 (A,D), E13.5 (B,E) and E18.5 (C,F) control (co; A-C) and Prox1Nkx (D-F) whole-mount (A,B,D,E) and sections through (C,F) isolated mouse hearts. At E10.5, Prox1Nkx cardiomyocytes are developing normally and the appropriate ultrastructure is laid down (A,D). From E13.5 onwards, Prox1Nkx cardiomyocytes remain as small rounded cells that do not acquire the characteristic rod shape (arrowheads; E,F). (G-J) In situ hybridisation for Nppa transcripts on frontal sections of E13.5 control (G,I) and Prox1Nkx (H,J) embryos. There is greatly reduced Nppa expression in Prox1Nkx myocardium (H,J). lv, left ventricle; rv, right ventricle; ra, right atrium. (K) The reduced Nppa expression in Prox1Nkx myocardium is confirmed by qRT-PCR on E12.5 isolated hearts. β-MHC (Myh7) was also found to be downregulated. (L) Morphometric analysis of cell shape (using ImageJ) confirmed a lack of increase in cell size because of impaired elongation and hypertrophic growth in Prox1Nkx cardiomyocytes during development, excluding the possibility that the rounded cells simply reflect an alteration in cell shape. In K,L, mean ± s.e.m.; *P<0.001, **P<0.003, ***P<9×10-7 (K), ***P<7×10-8 (L), ****P<3×10-12. (M) Foetal cardiomyocyte hypertrophic growth throughout normal development and in the absence of Prox1, where sarcomere striation is lost, myofibrils do not align and cardiomyocytes do not grow by hypertrophy. Green dotted lines, striated myofibrils; solid green lines, failed striation; blue ovals, nuclei. Scale bars: 10 μm in A-F; 50 μm in G,H; 20 μm in I,J.