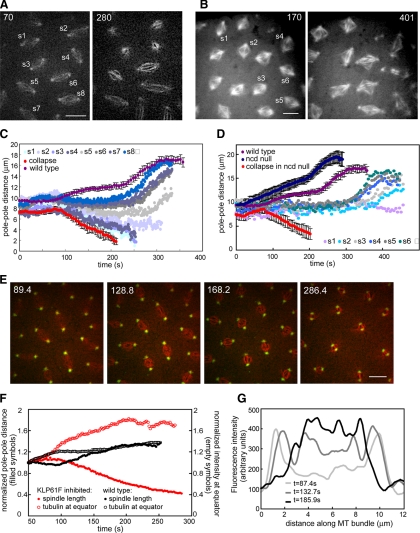
Figure 2.
(A) Two time points from a wild-type embryo injected with anti-KLP61F antibody showing a gradient of defects (see Supplemental Video 2). Bar, 10 μm. (B) Two time points from an Ncd null embryo injected with anti-KLP61F antibody showing more disorganized spindles due to the loss of two motors. Bar, 10 μm. (C) Graph of pole–pole distance as a function of time for the embryo shown in A. Close to the injection site spindles collapse (see s1 and s3), and other spindles shorten but remain small (see s2). Further away from the injection site, spindles do not elongate during prometaphase (s5, and the most distal spindles exhibit anaphase elongation (s6, s7, and s8). For comparison, we show graphs from a wild-type embryo (purple) and most severe effect (an embryo injected with enough antibody to collapse all spindles within the field of view; red). (D) Spindle length as a function of time for the Ncd null embryo shown in B. For comparison graphs of wild-type embryos (purple), Ncd null embryos (dark blue), and Ncd null embryos injected with a high concentration of anti-KLP61F (red) are shown. The gradient of concentration of antibody leads to a gradient of spindle pole lengths. A high inhibition of KLP61F leads to spindle collapse; lower concentrations lead to less severe defects. At a certain concentration the spindle maintains a steady length (light purple). (E) Anti-KLP61F antibody causes spindle collapse at prometaphase without apparent disruption of centrosomes or spindle poles. Images from time-lapse movie of an embryo expressing GFP-CNN (green) and injected with rhodamine tubulin (red) and anti-KLP61F (see Supplemental Video 3). Time in each frame is given in seconds. Bar, 10 μm. Note how the centrosomes move toward each other, but do not appear disorganized. (F) Tubulin fluorescence in the central spindle increases as the spindle collapses. Normalized spindle length (filled symbols) and fluorescent tubulin intensity at the equator (empty symbols) as a function of time for wild-type (black) and KLP61F inhibited spindles (red). After KLP61F inhibition spindles collapse with a correlated increase in tubulin intensity, suggesting that MTs are sliding inward and not depolymerizing. The increase is much higher than that observed in wild-type spindles, indicating that it is not due to a normal increase in tubulin. (G) Linescans of fluorescent tubulin intensity along a single ipMT bundle at different time points during the collapse. The intensity increases as the spindle collapses.