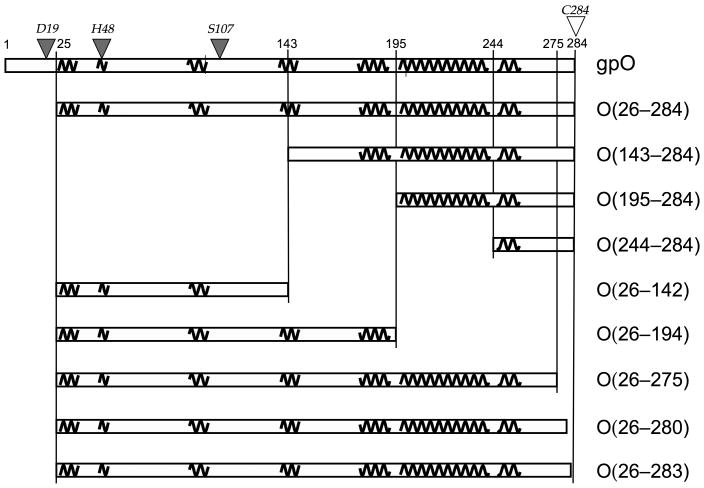
Figure 1.
Design of gpO truncation constructs. The sequence of gpO from 1–284 is represented by the rectangular box at the top. Pertinent residue numbers are shown. The predicted α-helical structures are shown as squiggly lines. Selected gpO truncation constructs that were made and the segments of α-helical structure included in each are shown in the boxes below. The filled triangles on top of the sequence indicate the residues that were shown by point mutations to correspond to the protease active site, while the C-terminal Cys residue that was shown by mutation to be essential for assembly is shown as an open triangle.