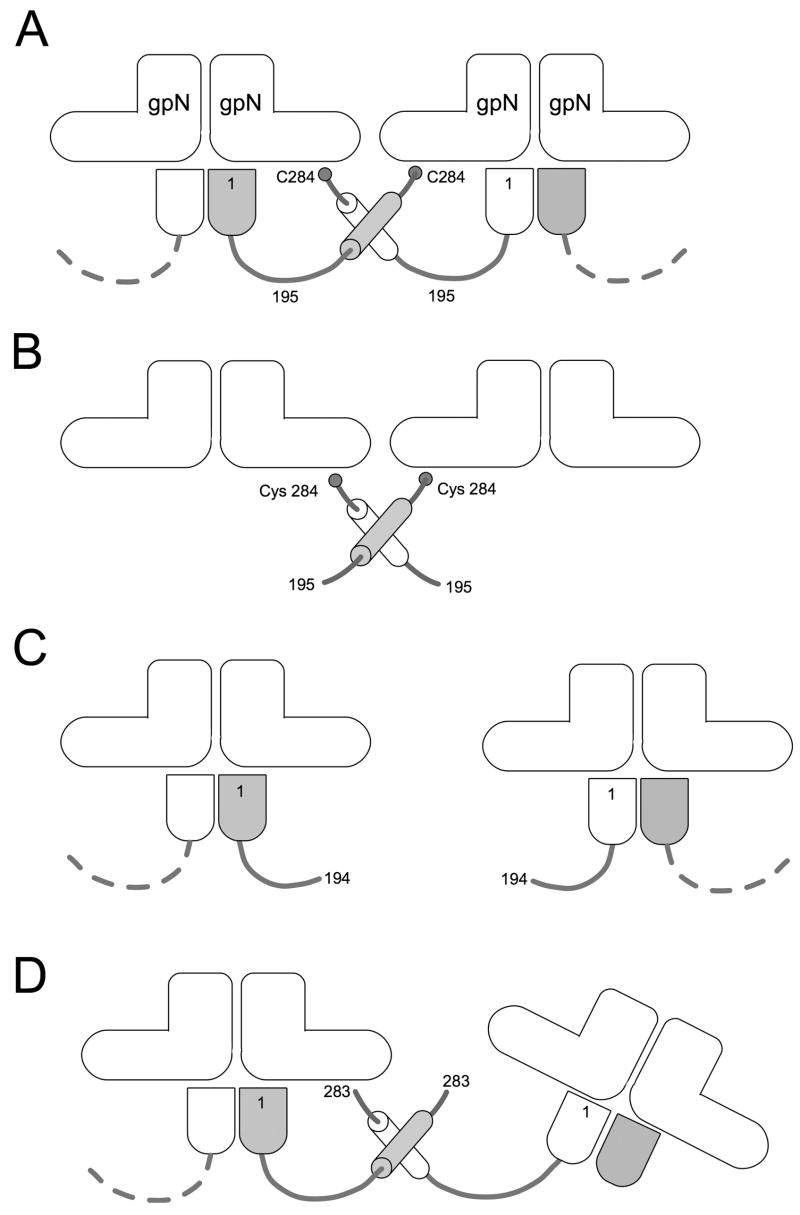
Figure 5.
Model for gpO action. The “L” shapes represent the gpN monomers. In the full-length gpO protein (A), the N-terminal protease domain (bullet shape) binds (as an oligomer) to the underside of gpN, while interactions between the α-helical domain (cylinders) and between Cys 284 and gpN lead to oligomerization and capsid assembly. In the absence of the N-terminal domain (B), O(195–284) is sufficient to promote correct assembly. O(26–194), however, lacks the required C-terminal domain, and assembly fails (C). The removal of only the C-terminal Cys 284 affects the fidelity of assembly and the size distribution of the procapsids (D). In this case, however, interactions between the α-helical domains can still occur, and about 55% of the shells formed are isometric.