Table 1.
Ruthenium-catalyzed ring-expansion of silyl-substituted alkynylcyclopropanols
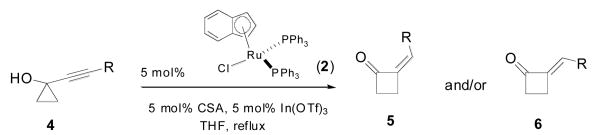 | ||||
|---|---|---|---|---|
| Entry | R | Z/E (5/6) ratioa | Time(h) | Yieldb |
| 1 | TMS 4a | 5.7:1 | 2 | 98% |
| 2 | BDMS 4b | 6.0:1 | 4 | 94% |
| 3 | SiMe2Ph 4c | 6.0:1 | 2 | 96% |
| 4 | TES 4d | 10.0:1 | 2 | 97% |
| 5 | TBS 4e | 11.4:1 | 2 | 98% |
| 6 | TIPS 4f | > 20:1 | 2 | 87%c |
Geometry was assigned by analogy to the Z and E isomers 5a/6a: see Supporting Information for details.
Total yield of two isomers determined by 1H-NMR with mesitylene as internal standard.
Isolated yield. BDMS = benzyl(dimethyl)silyl.
