Abstract
TREK-2 expressed in mammalian cells exhibits small (∼52 pS) and large (∼220 pS) unitary conductance levels. Here we tested the role of the N-terminus (69 amino acids long) in the control of the unitary conductance, and role of the alternative translation initiation as a mechanism that produces isoforms of TREK-2 that show different conductance levels. Deletion of the first half (Δ1–36) of the N-terminus had no effect. However, deletion of most of the N-terminus (Δ1–66) resulted in the appearance of only the large-conductance channel (∼220 pS). In support of the critical function of the distal half of the N-terminus, the deletion mutants Δ1–44 and Δ1–54 produced ∼90 pS and 188 pS channels, respectively. In Western blot analysis, TREK-2 antibody detected two immunoreactive bands at ∼54 kDa and ∼60 kDa from cells expressing wild-type TREK-2 that has three potential translation initiation sites (designated M1M2M3) within the N-terminus. Mutation of the second and third initiation sites from Met to Leu (M1L2L3) produced only the ∼60 kDa isoform and the small-conductance channel (∼52 pS). Mutants designed to produce translation from the second (M2L3) or third (M3) initiation site produced the ∼54 kDa isoform, and the large conductance channel (∼185–224 pS). M1L2L3, M2L3 and M3 were relatively selectively permeable to K+, as judged by the 51–55 mV shifts in reversal potential following a 10-fold change in [K+]o. PNa/PK values were also similar for M1L2L3 (∼0.02), M2L3 (∼0.02) and M3 (∼0.03). Arachidonic acid, proton and membrane stretch activated, whereas dibutyryl-cAMP inhibited all three isoforms of TREK-2, indicating that deletion of the N-terminus does not abolish modulation. These results show that the small and large conductance TREK-2 channels are produced as a result of alternative translation initiation, producing isoforms with long and short N-termini, and that the distal half of the N-terminus controls the unitary conductance.
TREK-2 (K2P10.1) is a member of the two-pore domain K+ channel family, and is expressed in neurons and glial cells in various areas of the central nervous system (Bang et al. 2000; Lesage et al. 2000; Gnatenco et al. 2002; Gu et al. 2002). In these cells, TREK-2 probably provides part of the background K+ conductance, and regulates the resting membrane potential and firing frequency. Because of its sensitivity to various factors such as lipids, membrane tension, acid, anaesthetics and G proteins, TREK-2 is likely to regulate neuronal excitability in response to diverse physiological and pathological stimuli (Patel et al. 1998, 1999, 2001; Kim, 2005; Honore, 2007). For example, expression of TREK-2 in dorsal root ganglion neurons and in the granular cells of the cerebellum suggests a role in sensory and pain transduction, and motor coordination, respectively.
Cloned rat and human TREK-2 expressed in COS-7, HEK293, HeLa cells and in Xenopus oocytes show multiple unitary conductance levels that range from ∼52 pS to ∼220 pS (Kang et al. 2007). The 52 pS channel shows a mild inwardly rectifying current–voltage relationship, whereas the 220 pS channel shows a linear relationship. Such a large difference in unitary conductance levels for a channel is unusual. The finding that TREK-2 mRNA produces channels with multiple conducting levels initially suggested that low conductance levels represent subconductance states of the large conductance level, or that small and large levels represent different gating modes, as also suggested initially for TREK-1 (Xian Tao et al. 2006). However, such mechanisms do not seem likely for TREK-2, because no switching of the 52 pS and 220 pS channels has ever been observed in patches containing only one conductance level. It also seems unlikely that the 52 pS channels are clustered and gate together to produce the 220 pS channel, because the open channel noise of the large-conductance channel is much lower than that of the small-conductance channel (Kang et al. 2007). Multiple openings of the 52 pS channels with added open channel noise are often observed in patches, ruling out the clustered gating mechanism. What other mechanism could possibly produce channels with such different conductance levels? In our preliminary studies to further understand this phenomenon, we observed that the deletion of the N-terminus of TREK-2, but not the C-terminus, produced only the large-conductance 220 pS channel. This finding suggested that the N-terminus controls the unitary conductance of TREK-2, and led us to hypothesize that small- and large-conductance levels may represent different conformation of the TREK-2 with the N-terminus in various locked positions formed during channel assembly at the plasma membrane, or that TREK-2 with truncated N-terminus is formed from the wild-type TREK-2.
During the course of our investigation to further determine the role of the N-terminus on TREK-2 function, a study reported that an alternative translation initiation mechanism produced two isoforms of TREK-1 (Thomas et al. 2008). This mechanism is thought to occur when the ribosome skips the first translation initiation site (referred to as leaky scanning), because of lower than optimal context of the sequences near the initiation codon AUG, and moves to the next initiation site to start translation (Kozak, 1989, 2005). Another important finding was that the isoform with the truncated N-terminus was permeable to Na+, leading to the idea that one of the TREK-1 isoforms can be excitatory (Thomas et al. 2008). These observations in TREK-1 have led us to further explore the possibility that an alternative translation initiation mechanism also produces TREK-2 isoforms with long and short N-termini that produce different conductance levels. In addition to the role of the N-terminus in the control of unitary conductance, functional properties of TREK-2 isoforms were studied.
Methods
Transfection in cloned cell lines
Full length rat TREK-2, TREK-1 and TRAAK were cloned into pcDNA3.1 vector (Invitrogen, Carlsbad, CA, USA). HeLa and COS-7 cells were seeded at a density of 2 × 105 cells per 35 mm dish 24 h prior to transfection in 10% bovine serum in Dulbecco's modified Eagle's medium (DMEM). Cells were cotransfected with pcDNA3.1 vectors harbouring full open reading frames of a K2P channel and green fluorescent protein (GFP) using LipofectAMINE 2000 and OPTI-MEM I Reduced Serum Medium (Life Technologies). Green fluorescence from cells expressing GFP was detected with the aid of a Nikon microscope equipped with a mercury lamp light source. Cells were used 1–3 days after transfection.
TREK-2 mutants
Single and double point mutations were done using QuikChange site directed mutagenesis kit (Stratagene) in pcDNA3.1. N-terminus deletion mutants were made by amplification of the desired DNA fragment and cloning into pcDNA3.1 vector by TA cloning methods. The DNA sequences of all TREK-2 mutants were sequenced for confirmation (DNA sequencing facility, University of Chicago).
Electrophysiological studies
Electrophysiological recording was performed using a patch clamp amplifier (Axopatch 200, Axon Instruments, Union City, CA, USA). Single channel current was filtered at 2 kHz using an 8-pole Bessel filter (−3 dB; Frequency Devices, Haverhill, MA, USA) and transferred directly to a computer using the Digidata 1320 interface (Axon Instruments) at a sampling rate of 20 kHz. Whole cell currents were recorded after cancelling the capacitive transients. Channel currents were analysed with the pCLAMP program (v. 9). For single channel analysis, the filter dead time was 100 μs (0.3/cutoff frequency) such that events shorter than 52 μs in duration would be missed.
Data were analysed to obtain channel activity (NPo, where N is the number of channels in the patch, and Po is the probability of a channel being open). NPo was determined from ∼30 s of current recording.
The single channel current tracings shown in the figures were filtered at 1 kHz. In experiments using cell-attached and excised patches, pipette and bath solutions contained (mm): 140 KCl, 1 MgCl2, 5 EGTA and 10 Hepes (pH 7.3). In other cell-attached and whole-cell recordings, bath solution contained (mm): 135 NaCl, 5 KCl, 1 MgCl2, 5 glucose and 10 Hepes (pH 7.3). A large outside-out patch was formed from the whole-cell configuration by gently lifting up the pipette.
For statistics, Student's t test was used with P < 0.05 as the criterion for significance. Data are represented as mean ±s.e.m. unless specified.
PNa/PK was calculated from ([K+]i/[Na+]o) exp(ΔVrevF/RT); where ΔVrev represents the change in Vrev on replacing K+ with Na+ in the bath solution, and F/RT is 0.039 mV−1 at 24°C.
Western blot analysis and reagents
TREK-2 antibody (Santa Cruz) recognizing the C-terminus of TREK-2 was used to perform Western blot analysis in cells expressing TREK-2 or its mutants. Briefly, cells in culture or brain tissues were collected and solubilized in RIPA buffer containing 52 mm Tris-HCl (pH 8.0), 150 mm NaCl, 1% NP-40, 0.5% sodium deoxycholate and 0.1% SDS. After mixing with sample buffer, proteins were separated by SDS-PAGE on a NuPAGE 4–12% gradient gel and transferred to a PVDF paper. Primary TREK-2 antibody raised in goat and secondary HRP-conjugated IgG antigoat antibodies were used to detect TREK-2 isoforms. The filter was exposed to Supersignal West Pico Chemoluminescent substrate (Pierce) for 5 min and then exposed imaging film (Kodak) for detection of HRP.
All other chemicals and enzymes were purchased from Sigma Chemical Co. Solutions for Western blot analysis were from Pierce. Antibodies to c-myc and HA epitopes were from Sigma. Dibutyryl-cAMP, arachidonic acid and isobutylmethylxanthine were purchased from Sigma.
Results
Small and large conductance levels of TREK-2
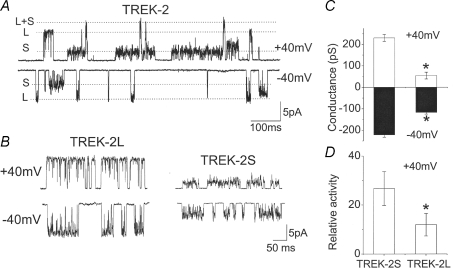
Rat TREK-2 is a 538 amino acid polypeptide, and two subunits are believed to assemble into a homodimer to form a functional K+ channel. When expressed in HeLa cells and single channels are recorded from cell-attached and inside-out patches, TREK-2 shows small and large channel conductance levels, as shown in Fig. 1A, as indicated by S (small; ∼52 pS) and L (large; ∼220 pS) recorded at +40 mV (outward current) and −40 mV (inward current). Channel openings with intermediate conductance levels (ranging from ∼120 pS to ∼180 pS) are also observed at lower frequency. One such opening with an intermediate conductance level is shown in the current tracing obtained at −40 mV in Fig. 1A (last opening). No such TREK-2 channels have been observed in patches from untransfected cells or in cells transfected only with DNA coding for green fluorescent protein (GFP), which is normally used to visualize cells expressing TREK-2. Small and large conductance levels of TREK-2 were also observed in COS-7 and HEK293 cells transfected with plasmid harbouring TREK-2 DNA. In cell-attached patches of HeLa cells with a low level of channel expression, channel openings with either small or large conductance level could be recorded (Fig. 1B). This allowed an accurate analysis of the current–voltage relationships for TREK-2-S and TREK-2-L (Fig. 1C). Thus, TREK-2-L has a linear current–voltage relationship, and TREK-2-S has an inwardly rectifying one. The channel activity of TREK-2-S was significantly greater than that of TREK-2-L in cell-attached patches (Fig. 1D).
Figure 1. Different conductance levels of TREK-2 expressed in HeLa cells.
A, current recordings from cell-attached patches. Pipette potential was held at +40 (cell membrane potential of ∼−40 mV) or −40 mV. S and L indicate small and large conductance levels indicated by dotted lines. B, current recordings from cell-attached patches containing only the small (TREK-2-S) or large (TREK-2-L) conductance channel. C, plot of the mean current amplitude at +40 and −40 mV (n = 5; mean ±s.e.m.). Asterisk indicates a significant difference (P < 0.05). D, relative channel activity of TREK-2-S and TREK-2-L measured from cell-attached patches (n = 10; mean ±s.e.m.). Asterisk indicates a significant difference from other bar (P < 0.05).
In those patches in which only TREK-2-S or TREK-2-L was present, no conversion of the conductance levels occurred during ∼30 min of recordings, suggesting that TREK-2-S is not a subconductance state of TREK-2-L, and that they do not represent different gating modes that can switch. Also, treatment of patches with arachidonic acid (10 μm), pressure (−60 mmHg) or acid (pH 5.8) all activated the channels as reported previously, but did not switch the gating mode (n = 6). Altering the temperature of the perfusion solution from 24°C to 35°C also did not alter the unitary conductance level of either TREK-2-L or TREK-2-S (n = 4, P > 0.05). These results suggest that TREK-2-L or TREK-2-S shows the properties of a preformed channel that does not undergo changes in gating mode or conformational switch.
Analysis of N-terminal deletion mutants
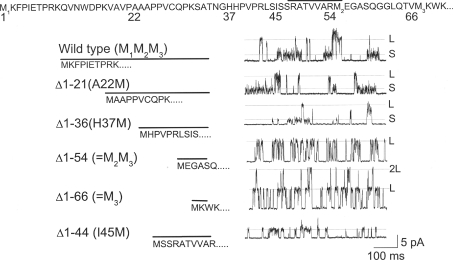
To identify channel regions that determine the unitary conductance of TREK-2, the effects of deletions of the cytoplasmic domains were tested. Our previous work showed that deletion of the C-terminus or substitution with the C-terminus with that of TASK-3 did not alter the unitary conductance level (Kim et al. 2001). Therefore, the effect of deletion of the N-terminus (69 residues) was examined. Based on secondary structure prediction programs (see figure legend), the N-terminus of TREK-2 consists of three domains (Fig. 2). The first domain consisting of residues 1–47 is predicted to be a random coil. The second domain consisting of residues from ∼48 to ∼56 (indicated by a line above the sequence) is predicted to be a short α-helix. The third domain is a short random coil consisting of ∼10 amino acids which connects to the first transmembrane α-helical segment. To test the role of these regions in controlling the unitary conductance level, six deletion mutants were constructed, and their single channel properties were studied.
Figure 2. Deletion of the N-terminus on the conductance level.
The amino acid sequence of the N-terminus (residues 1–69) is shown at the top. The region containing the residues 48–56 indicated by the line has a predicted α-helical structure, and the regions on either side of the α-helix are predicted random coil, based on secondary structure prediction programs (DPM, HNNC, Predator, nnPredict and PHDsec). Current tracings show single channel recordings from wild-type TREK-2 and deletion mutants, as indicated. For all deletions, the beginning amino acid was changed to methionine. Membrane potential was +40 mV. Small and large conductance levels are indicated by dotted lines (S and L).
The wild-type TREK-2 showed mainly small and large-conductance levels, with a few intermediate levels when recorded at +40 mV, as shown in the top tracing of Fig. 2. Deletions of up to ∼half of the N-terminus (Δ1–21:A22M, Δ1–32:A33M (not shown), and Δ1–36:H37M) did not affect the conductance levels, and both small and large levels were still present. Deletion of most of the N-terminus (Δ1–54 and Δ1–66) produced the large-conductance channel (ranging from 180 pS to 220 pS at +40 mV), and channels with small conductance levels were not observed. Deletion of the first 44 amino acids (Δ1–44:I45M) led to the appearance of a ∼90 pS channel that has less open channel noise than TREK-2-S in all 14 patches in both cell-attached and inside-out states (Fig. 2). This shows that the region encompassing residues 37–45 that is located before the putative α-helix is also necessary for producing the small-conductance (∼50 pS) channel. No large-conductance channel was observed with the Δ1–44:I45M mutant in all 14 patches tested. Together, these results provide further evidence that the entire distal half of the N-terminus controls the unitary conductance of TREK-2. The largest increase in unitary conductance (from ∼90 pS to ∼200 pS) occurs when the deletion is from residue 45–55, suggesting that the putative α-helical region is an important part of the channel that regulates the conductance level.
To test whether TREK-2-S can be switched to TREK-2-L by cleaving off the N-terminus, a thrombin cleavage site consisting of six amino acids (LVPRGS) was engineered into the position between residues 60 (Glu) and 61 (Gly). Unfortunately, no channels could be recorded from the mutant containing the six amino acids, suggesting that the proper length of the distal region of the N-terminus is important for formation of functional channels. Insertion of the same residues (LVPRGS) at a position between 45 (Ile) and 46 (Ser) led to normal small and large conductance channels (data not shown). These results further suggest that the proper conformation of the distal N-terminus is important for regulation of TREK-2 function and conductance.
Test of alternative translation initiation for TREK-2
A recent study reported that two isoforms of TREK-1 with different conductance levels are generated via an alternative translation initiation mechanism (Thomas et al. 2008). In this mechanism, the ribosomal complex can skip the first translation initiation site because of the suboptimal context of the sequences surrounding the starting codon (AUG), and start translating from the second initiation site. Therefore, it seems plausible that such a mechanism also accounts for the different conductance levels of TREK-2. The N-termini of TREK-1 and TREK-2 share 36% amino acid identity and 52% similarity, and have slightly different lengths (59 versus 69 residues). In TREK-2, there are three potential translation initiation sites at the N-terminus, as compared to two such sites for TREK-1. Theoretically, three isoforms can be produced for TREK-2 via the alternative translation initiation mechanism. The last two initiation sites are only 12 residues apart, and therefore the expected protein products from these initiation sites would be very close in molecular mass (∼53 and ∼54 kDa). If alternative translation initiation occurred, two protein products of ∼60 kDa and ∼53/54 kDa would be detected, based on translation from the first and second/third initiation sites.
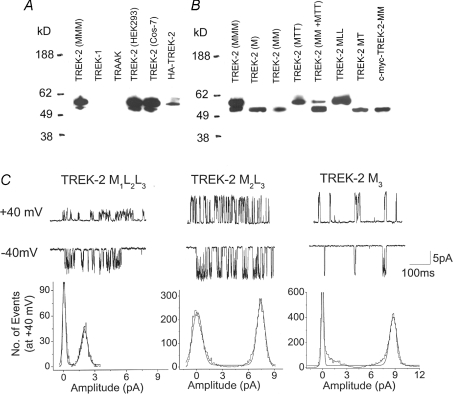
To test the generation of TREK-2 isoforms via alternative translation initiation, HeLa cells were transfected with TREK-2 DNA, and the expressed proteins were detected by Western blot analysis. As shown in Fig. 3A, two distinct immunoreactive bands were observed at the predicted molecular mass using an antibody directed against the C-terminus of TREK-2. No immunoreactivity was detected with cells expressing TREK-1 and TRAAK. Functional expression of TREK-1 and TRAAK in the cells was confirmed by single channel recording. Two bands at the same positions were also obtained when COS-7 and HEK293 cells expressing TREK-2 were used (Fig. 3A). The expression level of the ∼60 kDa isoform was always greater than that of the ∼54 kDa isoform. The relative density of the immunoreactive signals varied in different cultures, presumably due to different transfection efficiency of each plasmid at different times of transfection, as well as different efficiency of alternative translation (leaky scanning). Therefore, we focused only on the relative molecular sizes of the immunoreactive bands (isoforms). In HeLa cells expressing TREK-2 tagged with HA epitope at the N-terminus, the antibody to HA recognized only a single band at ∼60 kDa, as predicted.
Figure 3. Western blot analysis and single channel recordings of TREK-2 and its mutants.
A, cells expressing TREK-2, TREK-1, TRAAK or TREK-2 tagged with HA epitope were prepared for Western blot analysis. Two immunoreactive bands were detected using the TREK-2 antibody, whereas only one band was detected with the HA antibody. B, TREK-2, various mutants designed to elicit translation of initiation at specific sites were expressed in HeLa cells, and immunoreactive proteins were analysed with TREK-2 antibody. In cells expressing c-myc-tagged TREK-2, antibody to c-myc was used. C, single channel currents from cell-attached patches from HeLa cells expressing TREK-2 mutants, and the corresponding amplitude histograms (+40 mV) are shown.
For further test of the alternative translation initiation, mutants were constructed such that only one specific initiation site could be used. The wild-type TREK-2 will be referred to as M1M2M3, because it has three initiation sites at the N-terminus, indicated by the subscripts. First, the N-terminus up to either the second or the third initiation site was deleted to produce M2M3 (same as Δ1–54) and M3(same as Δ1–66), respectively. The second and third initiation sites (M2 and M3) were also mutated to leucine (or threonine) to generate M1L2L3 (or M1T2T3) and M2L3 (or M2T3) to prevent initiation from those sites. Western blot analysis again showed two immunoreactive bands at expected positions from HeLa cells expressing wild-type TREK-2. M3 and M2M3 produced only the smaller isoform, as predicted. M1T2T3 and M1L2L3 produced only the ∼60 kDa isoform, whereas M2T3 produced the ∼54 kDa isoform (Fig. 3B). M2T3 and M3 are close in molecular mass, and thus appeared similar in size. In cells transfected with both M2M3 and M1T2T3 DNAs, both isoforms were detected at predicted molecular sizes. The results show that leaky scanning occurs during translation of TREK-2, leading to the formation of the ∼60 kDa isoform and the ∼54 kDa isoforms. We were unable to separate the low molecular mass band to check for leaky scanning at the second initiation site. To show that TREK-2 isoforms are generated in brain tissues that express TREK-2 mRNA, cerebellum, trigeminal ganglia and hypothalamus were dissected from rat brain and subjected to Western blot analysis. In two separate experiments, no clear immunoreactive signals were obtained with the TREK-2 antibody. This suggests that the level of TREK-2 expression in these tissues may be relatively low for immunodetection, although single channels of TREK-2 can clearly be recorded from these tissues (Gnatenco et al. 2002; Han et al. 2002).
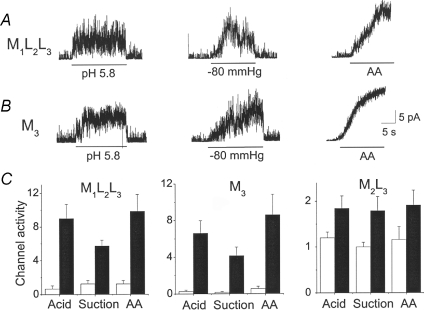
Single channels recorded from cell-attached patches of HeLa cells expressing M1L2L3, M2L3 or M3 show that M1L2L3 produces only the small conductance channel (52 pS), whereas M2L3 and M3 produce the large-conductance channel (185–220 pS; Fig. 2C). These results explain the presence of small- and large-conductance channels produced by TREK-2, and support the mechanism of alternative translation initiation. Amplitude histograms show a small difference in conductance between M2L3 and M3 at +40 mV. At +40 mV, the conductance levels of M2L3 and M3 were 188 ± 9 pS and 222 ± 8 pS, respectively. At −40 mV, the conductance levels were not different (216 ± 7 pS and 220 ± 6 pS; P < 0.05; n = 3). Therefore, M2L3 shows a very small inward rectification that is not present in M3.
As shown in Fig. 2, deletion of ∼half of the N-terminus (Δ1–36:H37M) still allowed leaky scanning, as both small and large-conductance channels were present. However, additional deletion of eight residues (Δ1–44:I45M) resulted in the generation of a channel exhibiting only the ∼90 pS channel, although Δ1–44:I45M has a suboptimal sequence at the first initiation site. It may be that the first initiation site of the Δ1–44:I45M mutant is now too close to the second and third sites for ribosomal escape to occur efficiently to elicit alternative initiation. We did not investigate this issue further.
Functional studies of TREK-2 isoforms: ion permeability to Na+ and K+
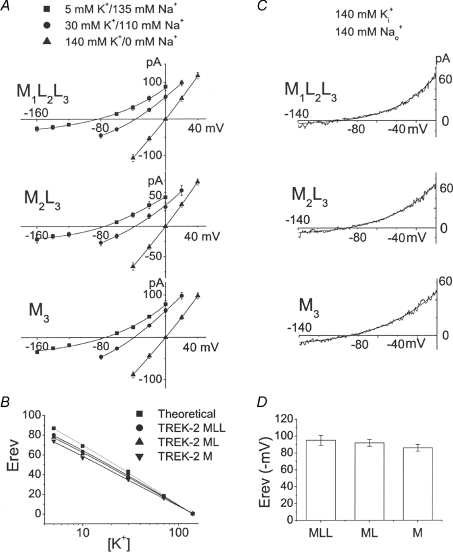
One of the findings with TREK-1 was that deletion of the N-terminus resulted in increased Na+ permeability (Thomas et al. 2008). To test whether the TREK-2 isoform with a short N-terminus also exhibits such a phenomenon, ion permeability studies were carried out using solutions containing varying ratios of K+ and Na+ concentrations. Large outside-out patches containing many channels were formed from HeLa cells expressing M1L2L3, M2L3 or M3, and macroscopic currents were recorded at various membrane potentials ranging from −160 mV to +40 mV. The initial bath and pipette solutions contained 140 mm K+/0 Na+, and then the bath solution switched to that containing 5 mm K+/135 mm Na+, 10 mm K+/130 mm Na+, 30 mm K+/110 mm Na+, or 70 mm K+/70 mm Na+. The current levels recorded at different voltages were plotted, and the reversal potentials were determined. As shown in Fig. 4A, lowering bath [K+] shifted the reversal potential (Erev) to the left as predicted of a K+-permeable channel. At a given [K+]o, the Erev values were generally similar for M1L2L3, M2L3 and M3 ranging from 75 mV to 82 mV. Plot of the Erev as a function of [K+] showed that the slope of the curves for M1L2L3, M2L3 or M3 were 55 ± 2, 54 ± 2 and 51 ± 2 mV (P < 0.05; n = 3) per 10-fold change in [K+]o, indicating that M1L2L3, M2L3 or M3 have similar ion selectivity and permeability properties (Fig. 4B). These results obtained using TREK-2 are clearly different from the ∼20 mV shift in Erev reported for TREK-1 after deletion of the N-terminus (Thomas et al. 2008).
Figure 4. Permeability properties of TREK-2 isoforms to K+ and Na+.
A, large outside-out patches were formed from cells expressing TREK-2 mutants each designed to produce one type of isoform. Pipette solution contained 140 mm K+, and the bath solution contained different ratio of K+ and Na+ as indicated. Arachidonic acid (5 μm) was added to the pipette to activate the channels. Macroscopic currents were determined at various membrane potentials and plotted as shown for M1L2L3, M2L3 and M3. B, shifts in reversal potential (Erev) are plotted as a function of external [K+], and fitted to a linear line. Dotted line indicates the calculated values from the Nernst equation. C, large outside-out patches with pipette that has 140 mm K+. Bath solution contains 140 mm Na+. D, the bar graph shows the reversal potentials for M1L2L3, M2L3 and M3 from experiments in C (n = 4; mean ±s.e.m.). No significant difference was present (P > 0.05).
To further confirm that Na+ permeability is relatively unchanged in isoforms with short N-terminus, currents from HeLa cells expressing M1L2L3, M2L3 or M3 were recorded with 140 mm Na+ in the bath and 140 mm K+ in the pipette solution, and then the bath solution was switched to that containing only 140 mm K+. Currents typical of those obtained following ramp voltage pulses ranging from −140 mV to 0 mV are shown in Fig. 4C. The three isoforms of TREK-2 showed Erev ranging from −86 mV to −96 mV (Fig. 4D). When Na+ in the bath was replaced with equimolar K+, the reversal potential was always zero in all trials (not shown). The calculated mean PNa/PK values were 0.02, 0.02 and 0.03 for M1L2L3, M2L3 or M3, respectively. These results show that the deletion of the N-terminus does not greatly alter the permeability properties of TREK-2 to Na+ and K+, although a small increase in Na+ permeability is seen with M3 compared with M1L2L3, M2L3. Again, this is different from the ∼10-fold increase in PNa/PK following removal of the N-terminus of TREK-1 (Thomas et al. 2008).
Functional studies of TREK-2 isoforms; activators and inhibitors
Having TREK-2 mutants each expressing only one isoform allowed us to directly test the effect of activators and inhibitors on the behaviour of each isoform. Therefore, inside-out patches from HeLa cells expressing M1L2L3, M2L3 or M3 were obtained, and arachidonic acid (10 μm), negative pressure (−60 mmHg) and acid (pH 5.8) were applied, and changes in channel activities determined before and after applications. As shown in Fig. 5A, all three activators strongly increased the channel activities of M1L2L3 from the basal level. Similarly, all three activators produced large increases in channel activity of M3 (Fig. 5B). Unlike M1L2L3 and M3 whose basal activity is relatively low, the basal activity of M2L3 was relatively high (open probability, ∼0.4). Because of this high basal activity, the increase in channel activity produced by application of the activators was relatively small, but the increase was clearly present (Fig. 5C). Comparison of the results obtained with M1L2L3 and M3 show that removal of the N-terminus does not affect the overall sensitivity of the channels to various activators.
Figure 5. Responses of TREK-2 isoforms to three activators.
A, inside-out patches expressing M1L2L3 were formed, and three activators (pH 5.8), suction (−80 mmHg) or arachidonic acid (20 μm) was applied briefly. Membrane potential is held at −40 mV. B, same experiments as A except that patches express M3. C, channel activities before and after activation are plotted for M1L2L3, M2L3 and M3. Each bar is the mean ±s.e.m. of 4–5 determinations. All three activators produced significant increases in activity from the corresponding basal level (P < 0.05).
To test the effect of phosphorylation, cell-attached patches were formed and 500 μm dibutyryl cAMP and 0.1 mm isobutylmethylxanthine (IBMX; inhibitor of phosphodiesterase) were applied to the bath solution to increase protein kinase A activity, which phosphorylates TREK-2 (Kang et al. 2006). Dibutyryl-cAMP and IBMX decreased the activity of M1L2L3, M2L3 and M3– to 36 ± 8%, 44 ± 9% and 42 ± 8% of the control level, respectively (n = 4; data not shown). However, unitary conductance levels were not affected (P < 0.05), and no switching of gating modes between small and large conductance levels was observed. Thus, deletion of the N-terminus did not alter the general behaviour of the TREK-2 channel in response to various modulators.
Intermediate conductance levels are sublevels of the large-conductance channel
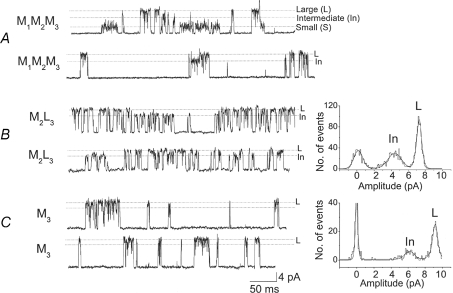
So far, we have focused on the molecular identity of small and large conductance levels of TREK-2. As described in an earlier study and in the first section of results, intermediate conductance levels are also observed with the wild-type TREK-2 (Kang et al. 2007). Single channel recordings from two cell-attached patches of HeLa cells expressing TREK-2 (M1M2M3) are shown in Fig. 6A. One can often observe channel openings that range from ∼120 pS to 150 pS (labelled as intermediate) along with the small (∼52 pS) and large (200–220 pS) conductance channels. In the middle of the second tracing of Fig. 6A, an intermediate level is present along with the large level, suggesting that the ∼150 pS channel is most likely a subconductance state of the ∼220 pS channel. However, it is difficult to know clearly whether the ∼150 pS channel is a subconductance level of the ∼220 pS channel, as the ∼150 pS channel could be a functional correlate of a different TREK-2 isoform.
Figure 6. Intermediate conductance levels of TREK-2 and its deletion mutants (M2L3 and M3).
Current recordings are from cell-attached patches at a holding membrane potential of ∼+40 mV. A, channel openings of wild-type TREK-2 show small, intermediate and large conductance levels indicated by dotted lines. B, channel openings of M2L3 show large and intermediate levels. Amplitude histogram obtained from the second tracing shows the ∼120 pS subconductance level. C, channel openings of M3 show mainly the large conductance level. Amplitude histogram shows the ∼150 pS subconductance level.
While studying TREK-2 mutants in more detail, we observed that homomeric channels made up of M2L3 frequently exhibit an intermediate level of ∼120 pS, in addition to the main conductance level ranging from ∼185 pS to ∼200 pS, as shown in the two tracings of Fig. 6B. To better assess the differences in conductance levels of large and intermediate conductance channels, two open levels (shown by dotted lines L and In) were used to obtain amplitude histograms. An amplitude histogram obtained from the second tracing in Fig. 6B where the intermediate levels are present frequently shows a population of channels with a mean conductance of ∼120 pS. For M3, the ∼120 pS intermediate conductance level was not observed, but the ∼150 pS level was present when two open levels (6.5 pA and 9 pA) were used to obtain the amplitude histogram (Fig. 6C). The rapid current fluctuation between the main ∼220 pS level and intermediate levels (∼150 pS to 200 pS) is typically observed in M3, and represents short-lasting subconductance levels as reported previously (Kang et al. 2007). M1L2L3 did not show any switching of the gating mode from the small to the intermediate level. Multiple openings of M1L2L3 showed greatly increased open channel noise and therefore could be clearly distinguished from the genuine intermediate levels observed with M2L3 and M3. These observations suggest that the ‘intermediate’ conductance levels (120 pS to 150 pS) observed with wild-type TREK-2 correspond to the subconductance levels of the low molecular mass isoforms of TREK-2. The appearance of subconductance levels produced by K+ channels has been explained by a mechanism of heteromeric pore conformations in which different subunits of the channel contribute differently to the pore function (Chapman & VanDongen, 2005), and such a mechanism probably applies to TREK-2 as well.
Discussion
TREK-2, a two-pore domain K+ channel, exhibits small and large conductance levels when expressed in mammalian cells. Because a typical ion channel produces mainly one open level with small differences in amplitude at a given membrane potential, the large difference in conductance levels (52 pS to 220 pS) observed with TREK-2 was very puzzling. Our studies here on the N-terminal deletion of TREK-2 and immunodetection of two forms of TREK-2 provide an explanation for the generation of small and large conductance levels by different isoforms produced by alternative translation initiation mechanism. Our findings in TREK-2 also support the recent observation of a similar mechanism that produces two isoforms of TREK-1 with different conductance levels (Thomas et al. 2008). However, clear functional differences are found between TREK-1 and TREK-2 isoforms with respect to Na+ permeability. The key finding of this study is that the high molecular isoform produced by translation from the first initiation site generates the small-conductance channel, whereas the small molecular isoform produced by translation from the second and/or third sites generates the large-conductance channel. Thus, the N-terminus where all the initiation sites are located controls the channel conductance of TREK-2.
Alternative translation initiation mechanism for TREK-2
Many years of work by Kozak shows that ATG codons can be in an optimal or a suboptimal context (Kozak, 1989, 2005). According to Kozak's scanning model of translation, several independently initiated proteins can be produced from one mRNA by context-dependent leaky scanning. The most optimal sequence for translation initiation is G−3CCATGG+4 or A−3CCATGG+4 where A in ATG is position 1. More generally it is (GCC)RCCATGG where R is a purine (A or G). The purine (G or A) at position −3 and G at position +4 are believed to be critical. Any change from this sequence leads to a weak context and < 10-fold efficiency in translation. Our study on TREK-2 and the recent work on TREK-1 clearly show that the alternative translation initiation mechanism accounts for the generation of isoforms with different unitary conductance levels for both channels. The TREK-2 clone used here has both intact 5′- (132 bases) and 3′-untranslated (∼600 bases) regions, as it was cloned from a rat brain cDNA library. In TREK-2, there are three potential initiation sites located at positions 1, 55 and 67 at the N-terminus, and the first ATG site (G−3CAATGA+4) is in a suboptimal context for translation initiation, according to the formulation of Kozak (1989). Therefore, this may be one of the factors that cause ribosomal skipping to occur. The initiation sequence at the second translation site is of optimal context, and therefore one would expect minimal ribosomal skipping to the third initiation site. Therefore, we predicted that the low molecular isoform of TREK-2 represents mainly the translation product from the second initiation site, although we do not have biochemical evidence supporting this interpretation. Regardless of whether the translation occurs from the second and/or third initiation site of TREK-2, our results show that the channels produced from these two initiation sites both show mainly the large-conductance levels, compared to the channel produced from the first initiation site.
One interesting observation was that M2L3, the product translated from the second initiation site, was more active than those of M1L2L3 and M3 in cell-attached patches. We also observed high basal activity with M2T3, indicating that replacement of methionine with leucine or threonine is not the cause of high basal activity. Such high basal activity of the large-conductance channel is not commonly observed in patches expressing wild-type TREK-2, although a few patches do show active large-conductance phenotype. Although the reason for the high basal activity of M2L3 and M2T3 is not clear, it suggests that the kinetic behaviour of the ∼220 pS TREK-2-L observed from the wild-type TREK-2 is more similar to M3 than to M2L3. Furthermore, M2L3 has a slightly inwardly rectifying current–voltage relationship (188 pS at +40 mV and 222 pS at −40 mV), whereas TREK-2-L and M3 show a linear current–voltage relationship. This would suggest that ribosomal skipping also occurs at the second initiation site, and translation from the third site occurs to produce the large ∼220 pS channel. Therefore, the lower molecular mass form detected by Western blot analysis may consist of two immunoreactive bands generated by initiation from both second and third sites. This will need to be confirmed in the future by direct sequencing of the isoforms.
The intermediate conductance levels of TREK-2 (∼120 pS to ∼150 pS) that are observed with the wild-type TREK-2 are likely to be the subconductance levels of lower molecular mass isoforms, as M2L3 and M3 show such intermediate levels. It is possible that heteromeric isoforms of TREK-2 made up of M1M2M3−M2M3, M1M2M3−M3 and M2M3−M3 also form to produce intermediate conductance levels. Future studies to identify all heteromeric forms of TREK-2 isoforms and their kinetic behaviour in heterologous and native systems will be necessary to better understand the biophysical properties of TREK-2.
The critical region of the N-terminus that controls unitary conductance
Studies using deletion mutants with different N-terminal lengths provide a clue as to which region may be critical in controlling the unitary conductance of TREK-2. Secondary structure prediction analysis of the N-terminus suggests that a short α-helix is located close, but not adjacent, to the beginning of the first transmembrane segment. The rest of the N-terminus has the properties of a random coil and therefore has little or no stable structure. The crystal structure of the N-terminus of KirBac1.1 also has a short α-helix located in a similar position (Kuo et al. 2003). This short α-helix in KirBac1.1 is referred as the ‘slide helix’, and is also found in other inwardly rectifying K+ channels. Our mutational analysis of the TREK-2 N-terminus indicates that the conductance level is controlled by the distal half of the N-terminus that contains the ‘α-helical’ region. Deletion of the N-terminus up to residue 37 (proximal half) has no effect, but deletion of seven additional residues (up to residue 44 that is located just before the α-helical region) results in a significant increase in conductance level from ∼52 pS to ∼90 pS. Deletion up to residue 55, which is at the end of the α-helical region, results in a 185–200 pS channel. Further deletion up to residue 67 results in ∼220 pS channel. Therefore, the entire distal half of the N-terminus is involved in the regulation of unitary conductance of TREK-2. The largest change in the conductance level (∼90 pS to ∼200 pS) occurs after the removal of the α-helical region, suggesting that this region is important for producing the small-conductance channel. This could potentially be accomplished by its interaction with other regions of the channel that affects the pore structure and function. In KirBac1.1, it has been speculated from the crystal structure that the slide helix region interacts with the proximal C-terminus, as well as with the first transmembrane segment (Kuo et al. 2003). It is also possible that the α-helix itself causes stabilization of the channel to produce a conformation that produces the small-conductance level by an interaction with the lipid membrane. In fact, such an interaction has been reported for the KirBac1.1 (Enkvetchakul et al. 2007), and altering the strength of interaction was found to increase the channel open probability. Whether channel conductance was also affected was not reported. In Kir2.1, mutations within the ‘slide helix’ region were found to cause one type of Andersen syndrome that is associated with periodic paralysis and cardiac arrythmia (Decher et al. 2007). Mutations within the slide helix region of Kir channels also produce inherited disorders (Schulte et al. 1999; Plaster et al. 2001; Gloyn et al. 2004). The interaction between the slide helix and a specific domain of the C-terminus facing the membrane was found to be important for normal gating of the channel. Perhaps, the proper conformation of the ‘slide helix’ region of TREK-2 is critical for the normal function of the channel, although its complete removal does not affect formation of a functional channel. This could explain why inserting six residues before, but not after, the α-helical region, produces a non-functional channel in TREK-2. Understanding of exactly how the N-terminus affects the pore conformation and the conductance levels of TREK-1 and TREK-2 will require further mutational analysis and the crystal structure of these channels.
TREK-2 isoforms have similar sensitivity to modulators, and similar ion permeability
Isoforms of a protein are generally produced to provide additional regulation to cell function. Therefore, a potential difference in sensitivity of the isoforms to activators or inhibitors of TREK-2 was initially suspected. However, our finding that all TREK-2 isoforms are similarly activated and inhibited by various modulators shows that modulation of TREK-2 does not require the presence of the N-terminus, but does not help us to understand why cells produce both forms of TREK-2. It is not surprising that the responses of the isoforms are similar, as all the activators and inhibitors act at the C-terminus of the channel (Honore et al. 2002; Murbartian et al. 2005; Honore, 2007). It has been suggested from the X-ray structure of KirBac1.1 that modulation of the channel by factors that act at the C-terminus could occur partly via interaction with the N-terminus (Kuo et al. 2003). Our results in TREK-2 show that the N-terminus is clearly unnecessary for such modulation. We have not studied in detail the difference in the level of expression of TREK-2 isoforms, but have noted that the channel expression of the isoform producing the small conductance channel (M1L2L3) in cell-attached and inside-out patches is always greater than the isoform producing the large, 220 pS conductance channel (M3). Therefore, it is conceivable that the background K+ current would be high in cells that express a higher ratio of M1M2M3 : M3 than in cells with a lower ratio. No such evidence has yet been obtained in the native tissues. We have not been able to detect TREK-2 expression by Western blot analysis in brain tissues presumably due to a low level of protein expression. However, both small- and large-conductance channels could be recorded in the same patches from dorsal root ganglion and cerebellar granule cells, indicating that alternative translation initiation occurs in these tissues (Han et al. 2002; Kang & Kim, 2006).
In our earlier study, phosphorylation of TREK-2 produced by activation of protein kinase A and C reduced the channel activity of small, intermediate and large levels of TREK-2 (Kang et al. 2007). Due to multiple conductance levels of TREK-2, it was not possible to clearly show whether phosphorylation caused a decrease in the frequency of opening and/or a shift in conductance level. Our results show that phosphorylation decreases the channel activity of each isoform, but does not affect the conductance levels.
In a recent study, the isoform of TREK-1 with truncated N-terminus was reported to be more permeable to Na+ than the isoform with the longer N-terminus (Thomas et al. 2008). This finding has led to the speculation that TREK-1 may be excitatory under certain conditions, and indeed cells expressing the TREK-1 isoform having a short N-terminus were more depolarized than cells expressing TREK-1 with long N-terminus (Thomas et al. 2008). TREK-2 isoforms did not show such behaviour, suggesting that the regulation of the pore function by the N-terminus is different for TREK-1 and TREK-2. Although TREK-1 and TREK-2 belong to the same subfamily of two-pore domain K+ channels and show similar responses to activators (arachidonic acid, acid and pressure) and inhibitors (Kim, 2003), the control of pore functions by the cytoplasmic domains may be different. Except for the proximal region of the C-terminus, the rest of the C-termini of TREK-1 and TREK-2 share very low amino acid identity, and could serve different functions in the regulation of ion permeability. Further studies are necessary to better understand the differences between TREK-1 and TREK-2 with respect to their pore function. The physiological significance of cells expressing two isoforms of TREK-2 with similar properties is difficult to know at this time. It is possible that the alternative translation initiation mechanism for TREKs is developmentally regulated to help with cell excitability during metabolic stress (which activates TREKs), such that large conductance channels are needed for fast inactivation, for example. Although many cells express different K+ channels with similar function (i.e. TASK1−3, IRK1−3), the precise role of each K+ channel in the regulation of cell excitation is still poorly understood, and is a topic of great interest and importance.
Acknowledgments
This work was supported by grants to D.K. (NIH HL-55363 and an American Heart Association grant-in-aid).
References
- Bang H, Kim Y, Kim D. TREK-2, a new member of the mechanosensitive tandem-pore K+ channel family. J Biol Chem. 2000;275:17412–17419. doi: 10.1074/jbc.M000445200. [DOI] [PubMed] [Google Scholar]
- Chapman ML, VanDongen AM. K channel subconductance levels result from heteromeric pore conformations. J Gen Physiol. 2005;126:87–103. doi: 10.1085/jgp.200509253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decher N, Renigunta V, Zuzarte M, Soom M, Heinemann SH, Timothy KW, Keating MT, Daut J, Sanguinetti MC, Splawski I. Impaired interaction between the slide helix and the C-terminus of Kir2.1: a novel mechanism of Andersen syndrome. Cardiovasc Res. 2007;75:748–757. doi: 10.1016/j.cardiores.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Enkvetchakul D, Jeliazkova I, Bhattacharyya J, Nichols CG. Control of inward rectifier K channel activity by lipid tethering of cytoplasmic domains. J Gen Physiol. 2007;130:329–334. doi: 10.1085/jgp.200709764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, Edghill EL, Frayling TM, Temple IK, Mackay D, Shield JP, Sumnik Z, van Rhijn A, Wales JK, Clark P, Gorman S, Aisenberg J, Ellard S, Njolstad PR, Ashcroft FM, Hattersley AT. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- Gnatenco C, Han J, Snyder AK, Kim D. Functional expression of TREK-2 K+ channel in cultured rat brain astrocytes. Brain Res. 2002;931:56–67. doi: 10.1016/s0006-8993(02)02261-8. [DOI] [PubMed] [Google Scholar]
- Gu W, Schlichthorl G, Hirsch JR, Engels H, Karschin C, Karschin A, Derst C, Steinlein OK, Daut J. Expression pattern and functional characteristics of two novel splice variants of the two-pore-domain potassium channel TREK-2. J Physiol. 2002;539:657–668. doi: 10.1113/jphysiol.2001.013432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J, Truell J, Gnatenco C, Kim D. Characterization of four types of background potassium channels in rat cerebellar granule neurons. J Physiol. 2002;542:431–444. doi: 10.1113/jphysiol.2002.017590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honore E. The neuronal background K2P channels: focus on TREK1. Nat Rev Neurosci. 2007;8:251–261. doi: 10.1038/nrn2117. [DOI] [PubMed] [Google Scholar]
- Honore E, Maingret F, Lazdunski M, Patel AJ. An intracellular proton sensor commands lipid- and mechano-gating of the K+ channel TREK-1. EMBO J. 2002;21:2968–2976. doi: 10.1093/emboj/cdf288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Choe C, Cavanaugh EJ, Kim D. Properties of single TREK-2 (K2P10.1) channels expressed in mammalian cells. J Physiol. 2007;583:57–69. doi: 10.1113/jphysiol.2007.136150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang D, Han J, Kim D. Mechanism of inhibition of TREK-2 (K2P10.1) by the Gq-coupled M3 muscarinic receptor. Am J Physiol Cell Physiol. 2006;291:C649–C656. doi: 10.1152/ajpcell.00047.2006. [DOI] [PubMed] [Google Scholar]
- Kang D, Kim D. TREK-2 (K2P10.1) and TRESK (K2P18.1) are major background K+ channels in dorsal root ganglion neurons. Am J Physiol Cell Physiol. 2006;291:C138–C146. doi: 10.1152/ajpcell.00629.2005. [DOI] [PubMed] [Google Scholar]
- Kim D. Fatty acid-sensitive two-pore domain K+ channels. Trends Pharmacol Sci. 2003;24:648–654. doi: 10.1016/j.tips.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Kim D. Physiology and pharmacology of two-pore domain potassium channels. Curr Pharm Des. 2005;11:2717–2736. doi: 10.2174/1381612054546824. [DOI] [PubMed] [Google Scholar]
- Kim Y, Gnatenco C, Bang H, Kim D. Localization of TREK-2 K+ channel domains that regulate channel kinetics and sensitivity to pressure, fatty acids and pHi. Pflugers Arch. 2001;442:952–960. doi: 10.1007/s004240100626. [DOI] [PubMed] [Google Scholar]
- Kozak M. The scanning model for translation: an update. J Cell Biol. 1989;108:229–241. doi: 10.1083/jcb.108.2.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozak M. Regulation of translation via mRNA structure in prokaryotes and eukaryotes. Gene. 2005;361:13–37. doi: 10.1016/j.gene.2005.06.037. [DOI] [PubMed] [Google Scholar]
- Kuo A, Gulbis JM, Antcliff JF, Rahman T, Lowe ED, Zimmer J, Cuthbertson J, Ashcroft FM, Ezaki T, Doyle DA. Crystal structure of the potassium channel KirBac1.1 in the closed state. Science. 2003;300:1922–1926. doi: 10.1126/science.1085028. [DOI] [PubMed] [Google Scholar]
- Lesage F, Terrenoire C, Romey G, Lazdunski M. Human TREK2, a 2P domain mechano-sensitive K+ channel with multiple regulations by polyunsaturated fatty acids, lysophospholipids, and Gs, Gi, and Gq protein-coupled receptors. J Biol Chem. 2000;275:28398–28405. doi: 10.1074/jbc.M002822200. [DOI] [PubMed] [Google Scholar]
- Murbartian J, Lei Q, Sando JJ, Bayliss DA. Sequential phosphorylation mediates receptor- and kinase-induced inhibition of TREK-1 background potassium channels. J Biol Chem. 2005;280:30175–30184. doi: 10.1074/jbc.M503862200. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Lesage F, Fink M, Romey G, Lazdunski M. Inhalational anesthetics activate two-pore-domain background K+ channels. Nat Neurosci. 1999;2:422–426. doi: 10.1038/8084. [DOI] [PubMed] [Google Scholar]
- Patel AJ, Honore E, Maingret F, Lesage F, Fink M, Duprat F, Lazdunski M. A mammalian two pore domain mechano-gated S-like K+ channel. EMBO J. 1998;17:4283–4290. doi: 10.1093/emboj/17.15.4283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AJ, Lazdunski M, Honore E. Lipid and mechano-gated 2P domain K+ channels. Curr Opin Cell Biol. 2001;13:422–428. doi: 10.1016/s0955-0674(00)00231-3. [DOI] [PubMed] [Google Scholar]
- Plaster NM, Tawil R, Tristani-Firouzi M, Canun S, Bendahhou S, Tsunoda A, Donaldson MR, Iannaccone ST, Brunt E, Barohn R, Clark J, Deymeer F, George AL, Jr, Fish FA, Hahn A, Nitu A, Ozdemir C, Serdaroglu P, Subramony SH, Wolfe G, Fu YH, Ptacek LJ. Mutations in Kir2.1 cause the developmental and episodic electrical phenotypes of Andersen's syndrome. Cell. 2001;105:511–519. doi: 10.1016/s0092-8674(01)00342-7. [DOI] [PubMed] [Google Scholar]
- Schulte U, Hahn H, Konrad M, Jeck N, Derst C, Wild K, Weidemann S, Ruppersberg JP, Fakler B, Ludwig J. pH gating of ROMK (Kir1.1) channels: control by an Arg-Lys-Arg triad disrupted in antenatal Bartter syndrome. Proc Natl Acad Sci U S A. 1999;96:15298–15303. doi: 10.1073/pnas.96.26.15298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D, Plant LD, Wilkens CM, McCrossan ZA, Goldstein SA. Alternative translation initiation in rat brain yields K2P2.1 potassium channels permeable to sodium. Neuron. 2008;58:859–870. doi: 10.1016/j.neuron.2008.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xian Tao L, Dyachenko V, Zuzarte M, Putzke C, Preisig-Muller R, Isenberg G, Daut J. The stretch-activated potassium channel TREK-1 in rat cardiac ventricular muscle. Cardiovasc Res. 2006;69:86–97. doi: 10.1016/j.cardiores.2005.08.018. [DOI] [PubMed] [Google Scholar]