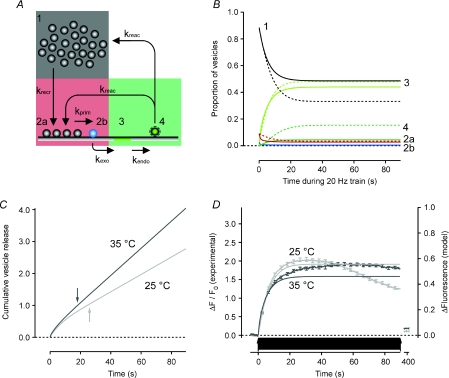
Figure 6. Expansion of the model to include endocytosis accounts for maintenance of the RRP during a train of APs at 20 Hz.
A, schematic illustration of the model. Vesicles are recruited from the reserve pool (1) to the RRP (2). Vesicles in the RRP become available for release after a priming reaction (2a and 2b) and are exocytosed to release transmitter (3) as in Fig. 5. Vesicle membrane at the surface is endocytosed (4) and will re-supply the RRP (10%) and the reserve pool (90%) after the pH gradient has been re-established. B, predictions of the model. Hatched lines are for 25°C (krecr= 0.074, kprim= 0.8, kexo= 11.5, kendo= 0.057 and kreac= 0.18 s−1) and continuous lines for 35°C (krecr= 0.079, kprim= 1.6, kexo= 8.6, kendo= 0.097 and kreac= 0.97 s−1). C, cumulative vesicle release rate at 25°C (light grey trace) and 35°C (dark grey trace) from the model. Note that the total pool of vesicles will be released several times at both temperatures. Arrows indicate time points when the total pool has been turned over once. D, predicted fluorescence intensity change (right axis) and measured sypHy fluorescence change (left axis) at 25°C (n = 430; light grey) and after 4 s at 35°C (n = 423; dark grey). Note that even though the steady state release at 35°C is higher than at 25°C the predicted fluorescence intensity is lower at the higher temperature, in line with the experimental data. Also note that the recorded fluorescence intensity at 25°C cannot maintain the expected steady state soon after the total vesicle pool has been turned over once (arrow in C).