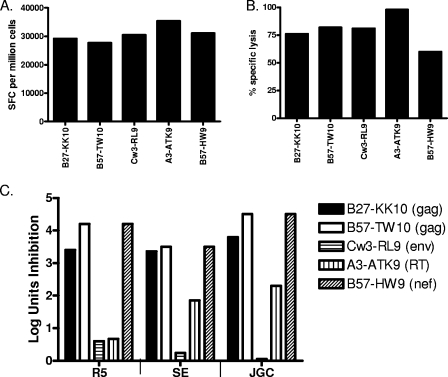
FIG. 2.
CD8 T-cell clones differ in antiviral efficacy depending on antigen specificity. (A and B) HIV-specific CD8 T-cell clones were isolated from HIV-infected individuals' peripheral blood by limiting dilution. The B27-KK10-specific clone was isolated from an elite controller with 1,060 SFC per million PBMCs assessed directly ex vivo in an IFN-γ ELISPOT assay, a B57-TW10-specific clone from a chronic progressor who did not recognize TW10 peptide in the IFN-γ ELISPOT assay at that time, a Cw3-RL9-specific clone from an elite controller with 1,360 SFC per million PBMCs, an A3-ATK-specific clone from an HIV-infected individual during a time the IFN-γ ELISPOT assay was technically not available, and a B57-HW9-specific clone from an elite controller with 1,640 SFC per million PBMCs. These clones were tested in a standard IFN-γ ELISPOT assay (A) and a 4-h chromium release assay with peptide-pulsed autologous or HLA-matched B-LCL targets (B). (C) The same clones were tested for antiviral function using autologous CD4 T cells infected with different primary HIV isolates. Peptide-specific CD8 T-cell clones differed in their antiviral efficacy with similar potency against the R5, SE, and JGC HIV isolates. Data are expressed as log inhibition units, calculated as −log10 (p24 with CTL/p24 without CTL) at day 7 in culture.