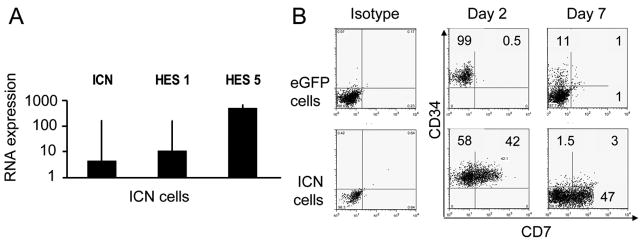
Figure 1. Notch1 induces CD7 on CD34+ UCB progenitors.
UCB CD34+ progenitors were transduced in Fibronectin coated transwells in the presence of c-kit-L, Flt3-L, IL-7 and TPO with MSCV-based eGFP and ICN vectors, FACS sorted for CD34+eGFP+ cells, plated in contact with murine embryonic liver stroma cell line EL08-1D2 with IL-3, c-kit-L, Flt3-L, IL-7 and IL-15 and cultured for 28 days. (A) The induction of specific mRNA from cultured ICN+ cells (■) and eGFP+ cells was analyzed by Q-RT- PCR. Relative transcripts of Notch1 (ICN) and downstream ICN targets Hes1 and Hes5 are relative to the level of GAPDH as a control for RNA integrity and reported as a ratio to eGFP controls. Bars represent mean expression ± SEM of 3 independent experiments (B) ICN+ and eGFP+ control cells were analyzed 2 days after transduction or after culture with EL08-1D2 stroma and cytokines (IL-3, c-kit-L, Flt3-L, IL-7, IL-15) for 7 additional days. All flow cytometry plots were gated on eGFP+ lymphocyte gate. Only ICN cell gave rise to a population of CD34+CD7+cells, which subsequently differentiated into CD34−CD7+ (and predominantly CD56−) lymphoid precursors.