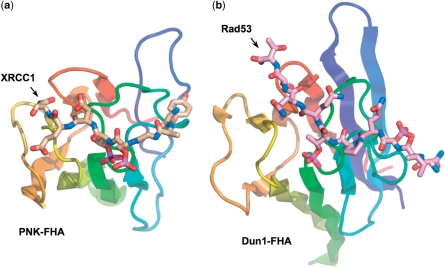
Figure 5.
Different modes of bis-phosphopeptide-binding in PNK and Dun1. The binding mode to PNK–FHA of the XRCC1 bis-phosphopeptide (a) with adjacent pSer-pThr residues is markedly different to that recently observed for a yeast Rad53 phosphopeptide containing two non-adjacent pThr residues, to the FHA of the DNA damage checkpoint kinase Dun1 (PDB code 2JQL) (b). While the C-terminal phosphorylated residue is in a similar topological position on the FHA domain, the N-terminal pThr in Dun1 binds in the −4 subsite, which is occupied by a tyrosine in PNK–FHA complexes with XRCC1 and XRCC4 peptides.