Fig. 1.
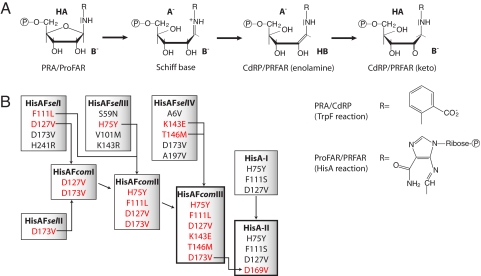
Selection and optimization of HisAF and HisA variants with TrpF activity. (A) Substrates, intermediates, and products of the reactions catalyzed by the TrpF and HisA enzymes. The reactions start with the protonation of the furanose ring oxygen of the aminoaldose (PRA or ProFAR) by a general acid (HA) and subsequent cleavage of the carbon-oxygen bond. Then, the resulting Schiff Base intermediate is deprotonated by a general base (B−) at the C2′ atom of its ribose moiety. The reaction product is the enolamine form of CdRP or PRFAR, which tautomerizes in an enzyme-independent manner to the corresponding aminoketose (15). The subsequent reactions in tryptophan and histidine biosynthesis, which are catalyzed by TrpC and HisF, yield indole glycerol phosphate and imidazole glycerol phosphate plus 5-aminoimidazole-4-carboxamide ribotide, respectively (33, 34). (B) Overview of the experimental workflow. The HisAFselI-IV variants isolated by in vivo selection contain the indicated amino acid exchanges. Bona fide beneficial exchanges of HisAFselI-IV (in red) were combined to generate HisAFcomI-III (for details, see the main text). Substitutions at positions 1–119 are located in the N-terminal half barrel of HisA, and substitutions at positions 120–250 are located in the C-terminal half barrel of HisF (Fig. S1). The earlier isolated HisA-I variant contains the 3 indicated exchanges (13). The additional D169V exchange, which corresponds to the equivalent D173V exchange within HisAFcomIII, was introduced into HisA-I to generate HisA-II. Residues lysine 143 and threonine 146 of HisAF are located within the C-terminal half barrel of HisF, in the long loop between β-strand 5 and α-helix 5 (Fig. S1). This loop has a different conformation in the C-terminal half barrel of HisA (22); therefore, no exchanges equivalent to K143E and T146M in HisAFcomIII could be identified and introduced into HisA-I. The 2 variants with wild-type like TrpF activity, HisAFcomIII and HisA-II, are framed in bold.