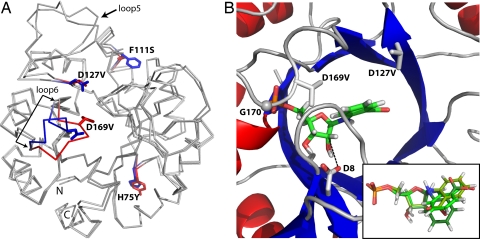
Fig. 3.
Crystal structure of HisA-II and modeling of PRA into its active site. (A) Comparison of the structures of HisA-II and wild-type HisA (PDB ID 1qo2). The 4 side-chain exchanges are indicated (blue sticks, wild-type HisA residues; red sticks, new residues present in HisA-II). The largest backbone difference is observed in loop β6α6, which carries the D169V exchange. Some variation can also be seen in the long loop β5α5; however, its conformation differs in the 2 molecules of the asymmetric units of both the HisA-II and the HisA structures illustrating its general flexibility. (B) Close-up of the active site of HisA-II with the best docking solution for PRA. A comparison of the top 3 PRA poses is shown in the inset [Glide scores XP-docking: −7.22 (green), −6.85 (pea), and −6.71 (forest)]. In HisA-II, glycine 170 (G170) (shown as sphere) forms a hydrogen bond with an oxygen atom of the phosphate group, which is enabled through a conformational change of loop β6α6 caused by the exchange D169V. The removal of negatively charged carboxylate groups caused by the exchanges D169V and D127V (new side chain shown as sticks) favour the accommodation of the negatively charged anthranilate moiety of PRA. The proximity of the wild-type residue aspartate 8 (D8) (shown as sticks) to the ribose moiety of PRA (distance between the closest carboxylate oxygen and the C2′ atom, 3.45 Å; indicated by broken line) suggests that it acts as the catalytic base of the TrpF reaction (Fig. 1A).