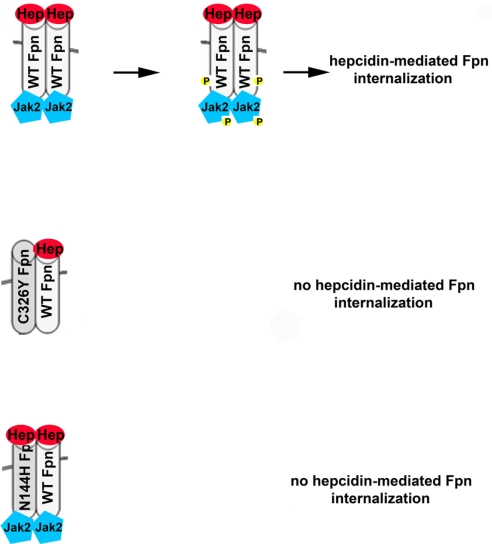
Fig. 7.
Model for hepcidin-resistant Fpn internalization. Fpn is a dimer and both monomers must bind hepcidin for Jak2 to bind to Fpn. Once Jak2 is bound, it is phosphorylated and then phosphorylates Fpn, which is the signal for Fpn internalization. Missense mutants of Fpn can participate in Fpn dimer formation. An inability to bind hepcidin (FpnC326Y) in one monomer will prevent Jak2 binding to a WT mutant heterodimer, resulting in retention of Fpn on the cell surface. Mutant Fpn that can bind hepcidin (FpnN144H) may lead to Jak2 binding; however, Jak2 is not activated and will not phosphorylate the WT mutant heterodimer. This results in retention of Fpn on the cell surface and continued iron export.