Abstract
The complete genome sequence of the crenarchaeon Ignicoccus hospitalis published recently in Genome Biology provides a great leap forward in the dissection of its unique association with another hyperthermophilic archaeon, Nanoarchaeum equitans.
A unique association in the archaeal world
The discovery of Nanoarchaeum equitans in a hydrothermal vent off the coast of Iceland by the group of Karl Stetter in 2002 has been one of the most exciting findings of the past decade in microbiology [1,2]. This tiny regular coccus (400 nm = 1% of the volume of Escherichia coli) lives at the surface (equitans meaning 'riding') of its host, the crenarchaeon Ignicoccus hospitalis (hospitalis referring to the ability to serve as a host for N. equitans), which belongs to the order Desulfurococcales within the Crenarchaeota (Figure 1). Members of the genus Ignicoccus are the only obligatory anaerobic chemolithoautotrophic sulfur reducers within the Desulfurococcales, coupling elemental sulfur respiration and carbon dioxide fixation through a novel and unique pathway called the dicarboxylate/4-hydroxybutyrate cycle and using molecular hydrogen as electron donor ([3] and references therein).
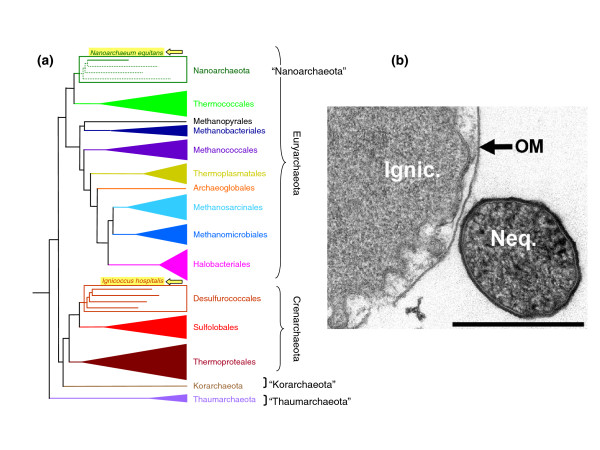
Figure 1.
(a) Schematic diagram of archaeal phylogeny showing the evolutionary relationships between archaeal lineages for which completely sequenced genomes are available (updated from [12]). The positions of Ignicoccus hospitalis and Nanoarchaeum equitans are indicated by arrows. The position of N. equitans has been controversial [2,8], but the position suggested here has been recently supported by a specific synapomorphy (a derived character state shared by two or more groups) [13] and signature genes [14]. (b) Electron micrograph showing a cell of N. equitans attached to a cell of I. hospitalis. The scale bar is 100 nm. Courtesy of Dr Rachel Reinhard, Regensburg University. OM: outer membrane.
Ignicoccus species exhibit irregular coccus morphology and are the only known archaeal cells to be surrounded by two membranes ([3,4] and references therein). The cytoplasmic membrane is separated from an outer membrane, which has a distinct lipid composition and contains pores of unique type, by a periplasmic space with a variable width of between 20 and 500 nm. The association between N. equitans and I. hospitalis is particularly interesting because it is the first (and so far the only) known example of a parasitic/symbiotic partnership involving two archaea, and moreover two hyperthermophilic organisms [1]. N. equitans cannot be cultivated in the absence of I. hospitalis, whereas the latter thrives well without its putative symbiont [1]. Although no benefits for the host can be detected in co-cultures, N. equitans and I. hospitalis are pioneering colonizers in deep-sea hydrothermal vents [5], and their association has been proposed to help these chemolithotrophs compete with heterotrophs in sulfide-rich environments. Often, the genome of only one of the two partners in a symbiotic association has been sequenced. That of N. equitans has already been sequenced [2] and, fortunately, the genome of I. hospitalis has now been published in Genome Biology by Podar et al. [3], allowing for the first time a comprehensive genomic analysis of this unique archaeal association.
A host with a highly reduced genome
The most important finding of Podar et al. is that of few but striking similarities between the genomes of I. hospitalis and N. equitans. First, both have highly reduced genomes: N. equitans harbors a compact genome of 490 kb encoding 552 genes [2], which represents the smallest known genome for an exosymbiont, whereas the genome of I. hospitalis is around 1.3 Mb, the smallest among free-living organisms [3]. As pointed out by Podar et al., even the combined gene complement of both genomes is significantly smaller than that of the average free-living bacteria or archaea [3]. The authors put forward the hypothesis that, as with N. equitans [6,7], the small size of the genome of I. hospitalis is a derived trait, resulting from a massive reduction that occurred after the divergence of this crenarchaeon from other members of the Desulfurococcales [3]. They estimate that around 500 genes corresponding to archaeal-specific clusters of orthologous genes (arCOGs) [7], including 19 genes otherwise common to all crenarchaeal genomes, have been lost in the I. hospitalis genome [3]. The authors put forward the hypothesis that these losses may be linked to the adaptation of Ignicoccus to a strict anaerobic and autotrophic lifestyle compared with its relatives.
Moreover, as in the case of N. equitans, the genome of I. hospitalis shows little operon-structure conservation, very few paralogs and no transposable elements, indicating that both genomes have been streamlined by extensive chromosomal rearrangements. This process has gone further in the smaller N. equitans genome [2], and it has been proposed that these features are primitive, leading to the hypothesis that this archaeon is a living fossil [8]. To us, the fact that N. equitans and I. hospitalis have these same features seems to suggest instead that both genomes evolved through a similar reduction process, in agreement with the position of N. equitans in archaeal phylogenies as an euryarchaeon possibly related to the Thermococcales [6] (Figure 1).
Expanded gene families
Interestingly, despite an evolutionary trend toward reduction, the genome of I. hospitalis exhibits expansions of specific gene families, notably those coding for proteins harboring domains involved in the formation of macromolecular complexes, such as WD40 repeats, CBS and V4R domains [3]. Moreover, the genome of I. hospitalis has the lowest arCOG coverage among the Crenarchaeota: an important fraction of its open reading frames (approximately 20%) cannot be affiliated to any arCOG [3].
The experimental characterization of these novel proteins will surely help to decipher at the molecular level the unique mechanisms responsible for the association between I. hospitalis and N. equitans. The expanded family of proteins with V4R domains will be especially interesting to study, because these proteins are homologs to the Bet3 subunit of the TRAPPI vesicle-tethering complex that is conserved in all eukaryotes [9]. Microscopy observations have consistently shown that both round and elongated membrane-coated vesicles are released from the cytoplasmic membrane in the periplasm of I. hospitalis and sometimes appear to come into close proximity to, and fuse with, the outer membrane ([4] and references therein). This has led to the hypothesis that these vesicles might be involved in transport of metabolites from the host to N. equitans. It will be important to determine whether proteins with VR4 domains indeed participate in the vesicle-trafficking system observed in I. hospitalis and if these vesicules are really involved in the interaction with N. equitans. Alternative hypotheses suggest that transport of metabolites or substrates between the two cells occurs through unusual structures observed at the contact point between them ([10] and references therein). These structures could involve some of the I. hospitalis-specific proteins revealed by the genome analysis.
Horizontal gene transfer in a minimalist system
Using phylogenetic methods, Podar et al. [3] have identified a number of genes that were probably acquired by horizontal gene transfer (HGT), either from bacteria (4%) or from Euryarchaeota (6%). Although limited in extent, some of these HGT events might have been important in permitting the combination of a streamlined genome with efficient metabolic strategies. For instance, I. hospitalis may use a transporter acquired from a euryarchaeon to import ammonium for nitrogen assimilation, a wise strategy in a highly reduced environment [9]. Similarly, I. hospitalis may use a sulfur/polysulfide reductase complex of bacterial origin in addition to the one normally found in Crenarchaeota [3].
Podar et al. [3] present an extensive description of I. hospitalis genes involved in genetic information processing, transport, central metabolism, respiration and energy metabolism. A major question is whether some of the genes for metabolites and energy production originally present in the N. equitans genome were transferred to the genome of I. hospitalis and the gene products are now being imported back into N. equitans. HGT from N. equitans to I. hospitalis and vice versa has apparently occurred, but on a very limited scale (only 13 such genes were identified), indicating that N. equitans should be able to import and use metabolites and energy (ATP) from I. hospitalis, as previously experimentally demonstrated in the case of lipids and amino acids [10].
What next?
The discovery of the unique N. equitans/I. hospitalis system has significantly increased our knowledge of the archaeal domain in terms of its diversity (the discovery of a new main lineage), ecology (association between two archaea) and genomics (highly reduced archaeal genomes). The sequencing of the genomes of both partners now provides valuable data for elucidating the nature of this association. Important biological questions remain to be answered, however: for instance, we would like to know how the cell cycles of N. equitans and I. hospitalis are coordinated. Another important question concerns the wide diversity of associations involving a nanoarchaeal partner. Up to now, the association between I. hospitalis and N. equitans has been described as highly specific because attempts to infect other species of Ignicoccus or other hyperthermophilic archaea with N. equitans have failed. However, in the past few years, molecular ecological studies have identified nanoarchaeal sequences in hot marine and terrestrial environments from geographically distant regions, suggesting that nanoarchaeota are widely distributed around the world. More surprisingly, nanoarchaeota have recently been reported from hypersaline mesophilic environments [11]. It will be extremely interesting to determine if these sequences correspond to free-living nanoarchaea or if they are symbiotic/parasitic cells that have established associations similar to that between N. equitans and I. hospitalis. In the latter case, the presence of nanoarchaea in halophilic and mesophilic environments might involve hosts other than I. hospitalis or Desulfurococcales, because no mesophiles are currently known in these lineages. The characterization of these uncultivated nanoarchaea as well as that of their hosts will surely bring answers to these questions.
Acknowledgments
Acknowledgements
We thank Dr Rachel Reinhard for useful comments and the micrograph in Figure 1b.
References
- Huber H, Hohn MJ, Rachel R, Fuchs T, Wimmer VC, Stetter KO. A new phylum of Archaea represented by a nanosized hyperthermophilic symbiont. Nature. 2002;417:63–67. doi: 10.1038/417063a. [DOI] [PubMed] [Google Scholar]
- Waters E, Hohn MJ, Ahel I, Graham DE, Adams MD, Barnstead M, Beeson KY, Bibbs L, Bolanos R, Keller M, Kretz K, Lin X, Mathur E, Ni J, Podar M, Richardson T, Sutton GG, Simon M, Soll D, Stetter KO, Short JM, Noordewier M. The genome of Nanoarchaeum equitans: insights into early archaeal evolution and derived parasitism. Proc Natl Acad Sci USA. 2003;100:12984–12988. doi: 10.1073/pnas.1735403100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podar M, Anderson I, Makarova KS, Elkins JG, Ivanova N, Wall MA, Lykidis A, Mavromatis K, Sun H, Hudson ME, Chen W, Deciu C, Hutchison D, Eads JR, Anderson A, Fernandes F, Szeto E, Lapidus A, Kyrpides NC, Saier MH, Jr, Richardson PM, Rachel R, Huber H, Eisen JA, Koonin EV, Keller M, Stetter KO. A genomic analysis of the archaeal system Ignicoccus hospitalis-Nanoarchaeum equitans. Genome Biol. 2008;9:R158. doi: 10.1186/gb-2008-9-11-r158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Junglas B, Briegel A, Burghardt T, Walther P, Wirth R, Huber H, Rachel R. Ignicoccus hospitalis and Nanoarchaeum equitans: ultrastructure, cell-cell interaction, and 3D reconstruction from serial sections of freeze-substituted cells and by electron cryotomography. Arch Microbiol. 2008;190:395–408. doi: 10.1007/s00203-008-0402-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCliment EA, Voglesonger KM, O'Day PA, Dunn EE, Holloway JR, Cary SC. Colonization of nascent, deep-sea hydrothermal vents by a novel Archaeal and Nanoarchaeal assemblage. Environ Microbiol. 2006;8:114–125. doi: 10.1111/j.1462-2920.2005.00874.x. [DOI] [PubMed] [Google Scholar]
- Brochier C, Gribaldo S, Zivanovic Y, Confalonieri F, Forterre P. Nanoarchaea: representatives of a novel archaeal phylum or a fast-evolving euryarchaeal lineage related to Thermococcales? Genome Biol. 2005;6:R42. doi: 10.1186/gb-2005-6-5-r42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova KS, Wolf YI, Sorokin AV, Koonin EV. Clusters of orthologous genes for 41 archaeal genomes and implications for evolutionary genomics of archaea. Biol Direct. 2007;2:33. doi: 10.1186/1745-6150-2-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Giulio M. Nanoarchaeum equitans is a living fossil. J Theor Biol. 2006;242:257–260. doi: 10.1016/j.jtbi.2006.01.034. [DOI] [PubMed] [Google Scholar]
- Podar M, Wall MA, Makarova KS, Koonin EV. The prokaryotic V4R domain is the likely ancestor of a key component of the eukaryotic vesicle transport system. Biol Direct. 2008;3:2. doi: 10.1186/1745-6150-3-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jahn U, Gallenberger M, Paper W, Junglas B, Eisenreich W, Stetter KO, Rachel R, Huber H. Nanoarchaeum equitans and Ignicoccus hospitalis: new insights into a unique, intimate association of two archaea. J Bacteriol. 2008;190:1743–1750. doi: 10.1128/JB.01731-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanueva A, Galada N, Baker GC, Grant WD, Heaphy S, Jones B, Yanhe M, Ventosa A, Blamey J, Cowan DA. Nanoarchaeal 16S rRNA gene sequences are widely dispersed in hyperthermophilic and mesophilic halophilic environments. Extremophiles. 2008;12:651–656. doi: 10.1007/s00792-008-0170-x. [DOI] [PubMed] [Google Scholar]
- Brochier-Armanet C, Boussau B, Gribaldo S, Forterre P. Mesophilic Crenarchaeota: proposal for a third archaeal phylum, the Thaumarchaeota. Nat Rev Microbiol. 2008;6:245–252. doi: 10.1038/nrmicro1852. [DOI] [PubMed] [Google Scholar]
- Urbonavicius J, Auxilien S, Walbott H, Trachana K, Golinelli-Pimpaneau B, Brochier-Armanet C, Grosjean H. Acquisition of a bacterial RumA-type tRNA(uracil-54, C5)-methyltransferase by Archaea through an ancient horizontal gene transfer. Mol Microbiol. 2008;67:323–335. doi: 10.1111/j.1365-2958.2007.06047.x. [DOI] [PubMed] [Google Scholar]
- Dutilh BE, Snel B, Ettema TJ, Huynen MA. Signature genes as a phylogenomic tool. Mol Biol Evol. 2008;25:1659–1667. doi: 10.1093/molbev/msn115. [DOI] [PMC free article] [PubMed] [Google Scholar]