Fig. 5.
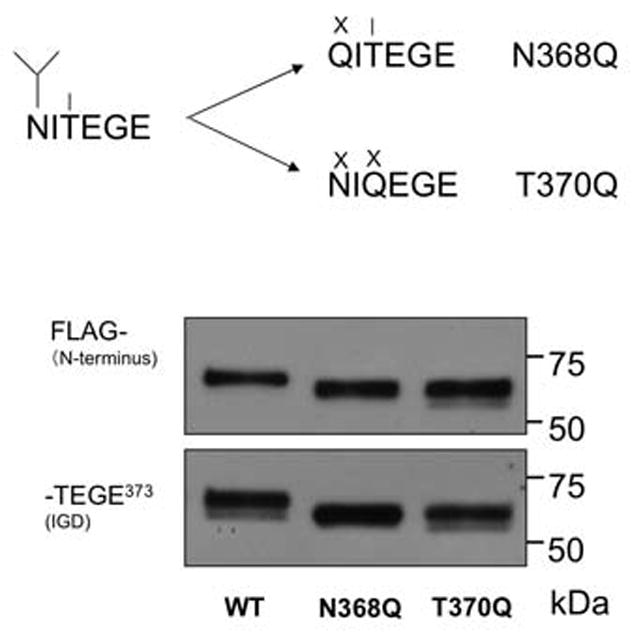
The effect of N-linked glycosylation at Asn368 on aggrecan cleavage by ADAMTS-4 (p40) at Glu373-Ala374. (A) Mutations at Asn368 and Thr370 remove a potential N-linked site (fork symbol). In N368Q the potentially O-glycosylated Thr (line symbol) remains available. (B) Mutation at Asn368 and Thr370 both result in reduction of the molecular mass of anti-FLAG and anti-NITEGE reactive fragment following digestion with ADAMTS-4 (p40) consistent with elimination of N-linked glycosylation at Asn368.
