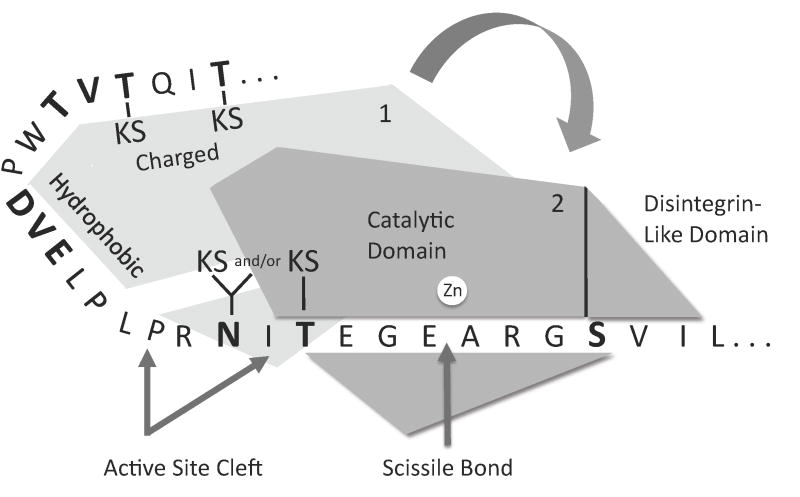
Fig. 9.
Model for interactions between aggrecan IGD and ADAMTS-4 or ADAMTS-5. Initial docking of the proteinase may occur via hydrophobic interactions between exosite residues and a region of the IGD remote from the cleavage site. In the second step, the active site cleft of the exosite-bound proteinase may dock with the cleavage site of the IGD substrate. Additional interactions may occur between Ser373 and the catalytic or disintegrin-like domain. Binding to the catalytic cleft is followed by chain scission at Glu373-Ala374 and dissociation of the exosite-bound and catalytic cleft-bound polypeptides. The indicated KS-substituted Thr and Asn residues are found in cartilage-derived aggrecan, and absent from recombinant aggrecan. Residues that were mutated in this study are shown in boldface.