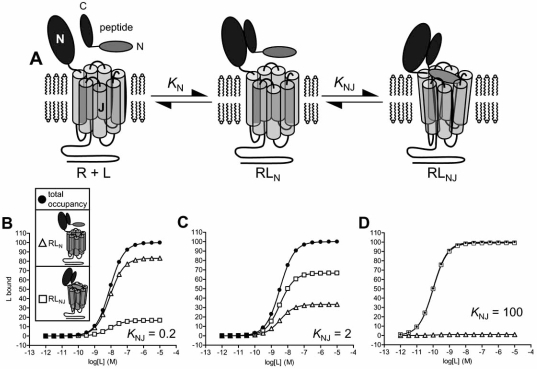
Fig. (1).
Two domain model of peptide interaction with Class B GPCR’s. A. Schematic representation of the two-domain model. The carboxyl-terminal portion of the peptide binds the extracellular N-terminal domain (N-domain) of the receptor, forming RLN, defined by the equilibrium constant KN. This interaction acts as an affinity trap, promoting interaction of the amino-terminal portion of the ligand with the juxtamembrane domain (J-domain) of the receptor, forming RLNJ. The J-domain interaction is defined by the isomerization constant KNJ, defining the RLNJ : RLN concentration ratio. B-D: Simulation of receptor occupancy, N-domain occupancy and J-domain occupancy by peptide ligand with varying strength of J-domain interaction. Occupancy of the receptor was defined by eq. 1 (Appendix), occupancy of the N-domain alone by eq. 2, and occupancy of the J-domain (with concomitant occupancy by the N-domain) by eq. 3. The parameter values used were: KN = 1×108 M-1, [R]TOTAL = 100, and values of KNJ of 0.2 (B, weak J-domain interaction), 2 (C, moderate interaction)and 100 (D, strong interaction).