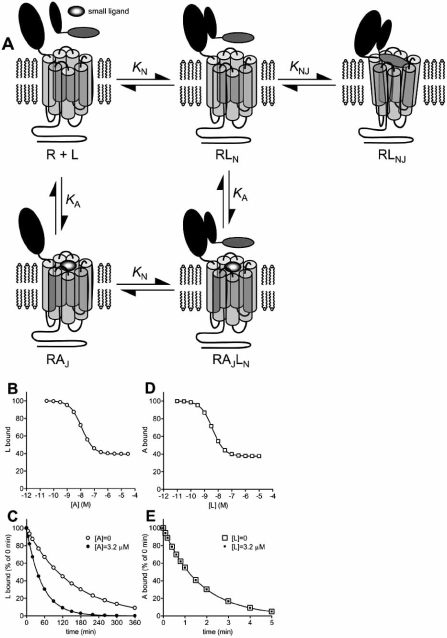
Fig. (4).
Charniere-type model of modulator interaction with Class B GPCR’s. A. Schematic representation of the model. In this model, a small ligand binds to one of the two sites of peptide interaction, in this specific example the J-domain. Binding of small ligand to this site, defined by the equilibrium constant KA, competitively inhibits peptide interaction with the J-domain but does not affect peptide binding to the N-domain. Note that in this model there is no allosteric interaction between the small ligand and peptide. B-E. Manifestation of the model in binding assays, simulated using equations in the Appendix. Binding parameters were: KN = 1×108 M-1, [R]TOTAL = 100, KNJ= 2, and KA =1×108. B. Modulation of equilibrium binding of peptide (L) by small ligand (A), simulated using eq. 13, [L] = 1×10-9 M. C. Modulation of peptide dissociation by small ligand, simulated using eq. 15. The dissociation rate of peptide from the N-domain (k-N) was set at 0.02 min-1. D. Modulation of equilibrium binding of small ligand by peptide ligand, simulated using eq. 17, [A] = 2×10-9 M. E. Modulation of small ligand dissociation by peptide, simulated using eq. 19. The dissociation rate of small ligand (k-A) was set at 0.6 min-1. Note that this model does not allow peptide to modulate dissociation of small ligand, in contrast to the allosteric model (Fig. 3E).