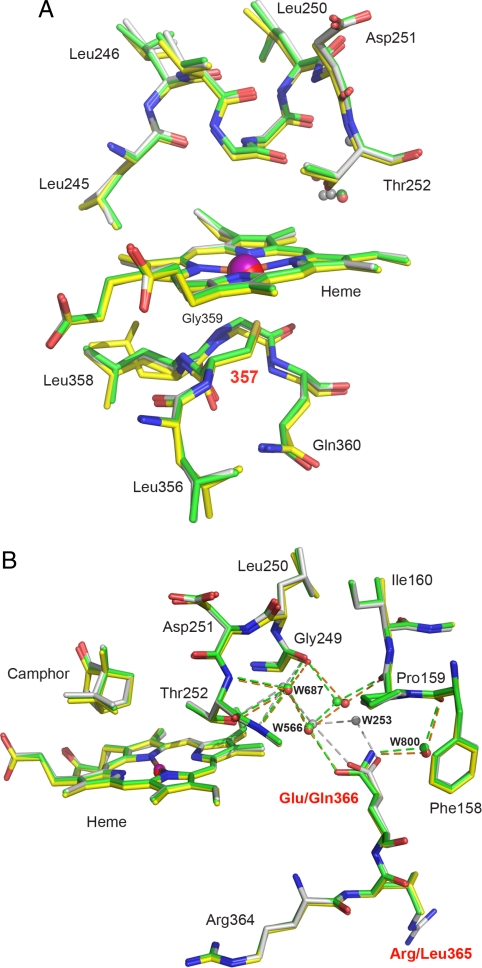
Fig. 3.
Structure of C357U P450cam* in the camphor-bound state. (A) Active site of the selenoenzyme (yellow) superimposed on that of P450cam* (green) and WT P450cam (1dz4, gray); all 3 structures were determined from the monoclinic crystal form. Although the axial cysteine ligand at position 357 was substituted by selenocysteine, the structural integrity of the enzyme is maintained. The increased Fe-Se distance may account for the 2 side chain conformations observed for Leu-358 in the selenoenzyme. The camphor-binding site is not affected by the mutations (see Fig. S2). The camphor molecule has been omitted for clarity. (B) View of the region around Gln-366 in C357U P450cam* (yellow), P450cam* (green), and the WT protein (gray). Mutating the native glutamate residue to glutamine induces displacement of the connected chain of ordered water molecules.