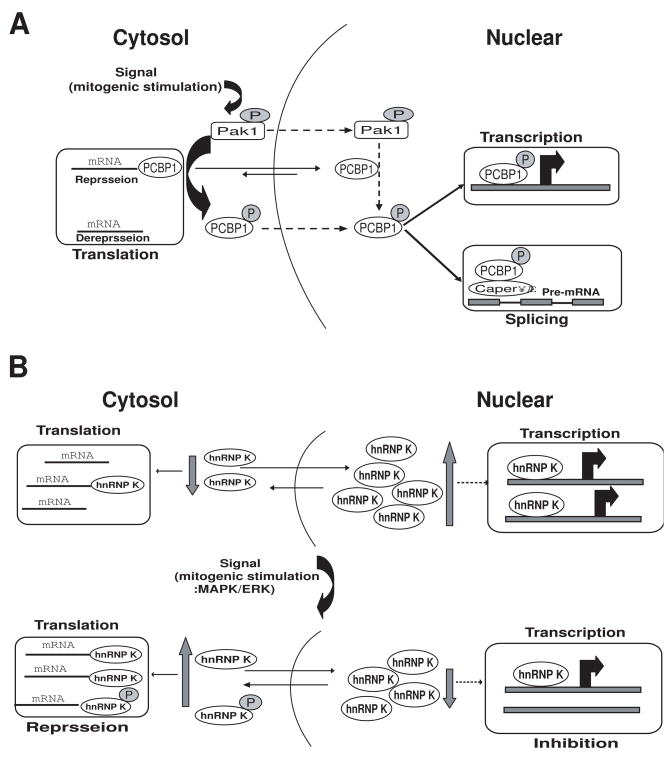
Fig. 3.
A, Proposed model for signal-dependent alterations in the nuclear and cytoplasmic activities of PCBP1. Upstream activators of the Pak1 pathway induce Pak1 kinase activity; active Pak1 has both cytoplasmic and nuclear functions. Pak1 phosphorylates cytoplasmic PCBP1, reducing its RNA-binding capabilities and thus releasing its translational repression of specific target mRNAs. Pak1 also phosphorylates PCBP1 in the nucleus (the mechanism by which phosphorylated PCBP1 moves from the cytoplasm to the nucleus is still not known). In the nucleus, PCBP1 is recruited to promoters on target genes and regulates their transcriptional activity. Phosphorylation also enhances PCBP1 binding to recently transcribed mRNA in the nucleus, influencing the splicing machinery via CAPER. Dashed lines represent the events that are not fully understood. Adapted from Meng et al., (2007) [23]. B, Proposed model for signal-dependent alterations of the nuclear and cytoplasmic activities of hnRNP K. MAPK/ERK efficiently phosphorylates hnRNP-K, increasing its accumulation in the cytoplasm and rendering the protein capable of regulating translation of mRNAs that have a DICE in the 3′UTR. Also, the quantity of hnRNP K in the can nucleus decrease, suggesting that the transcriptional activity of genes regulated by hnRNP K might also be changed. Dashed lines represent the events that are not fully understood. Adapted from Habelhah et al. (2001) [22].